Market Analysis:
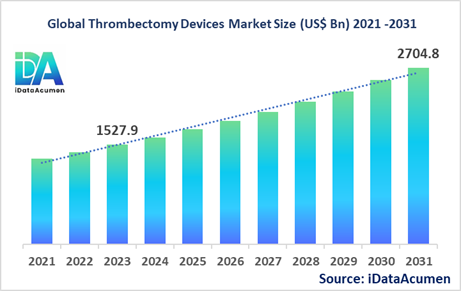
The Thrombectomy Devices Market had an estimated market size worth US$ 1,527.9 million in 2023, and it is predicted to reach a global market valuation of US$ 2,704.8 million by 2031, growing at a CAGR of 7.4% from 2024 to 2031.
Thrombectomy devices are medical instruments used to remove blood clots, also known as thrombi, from blood vessels. These devices are primarily used to treat conditions such as acute ischemic stroke, deep vein thrombosis, and peripheral artery disease, where blood clots can obstruct blood flow and lead to serious complications. The advantages of thrombectomy devices include improved patient outcomes, reduced recovery time, and decreased risk of complications compared to traditional surgical interventions.
The key drivers for the growth of the Thrombectomy Devices Market include the increasing incidence of stroke and other vascular disorders, advancements in thrombectomy device technology, growing awareness about the benefits of minimally invasive procedures, and rising demand for effective and time-sensitive clot removal treatments.
In summary, the Thrombectomy Devices Market is a rapidly growing segment within the medical devices industry, driven by the need for effective and minimally invasive treatments for various vascular disorders.
The Thrombectomy Devices Market is segmented by product type, end-user, application, and technology. By product type, the market is segmented into mechanical thrombectomy devices, aspiration thrombectomy devices, hybrid thrombectomy devices, rotational thrombectomy devices, and others (including ultrasonic and chemical thrombectomy devices). One of the largest and fastest-growing sub-segments is mechanical thrombectomy devices, which offer improved precision and efficiency in clot removal compared to traditional treatments.
Epidemiology Insights:
The global burden of vascular disorders, such as acute ischemic stroke and deep vein thrombosis, is significant and growing. In North America, the incidence of acute ischemic stroke is estimated to be around 800,000 cases per year, with a significant portion requiring thrombectomy intervention. Similarly, the prevalence of deep vein thrombosis in the United States is estimated to be around 900,000 cases annually.
The key epidemiological trends driving the growth of the Thrombectomy Devices Market include the aging population, increasing prevalence of risk factors such as obesity and diabetes, and improved diagnosis and awareness of vascular disorders. In major markets like the United States, the incidence of acute ischemic stroke is expected to continue rising due to the aging population and the increasing prevalence of underlying risk factors.
Growth opportunities in the Thrombectomy Devices Market are closely tied to the increasing patient population and the need for effective and timely interventions. As the incidence of vascular disorders continues to rise, the demand for advanced thrombectomy devices is expected to increase, particularly in regions with limited access to specialized stroke and vascular care.
It is important to note that certain vascular disorders, such as rare forms of peripheral artery disease, can be considered rare diseases, requiring specialized treatment approaches and targeted research and development efforts.
Market Landscape:
The Thrombectomy Devices Market is characterized by both unmet needs and ongoing advancements in treatment options. While traditional treatments, such as thrombolytic drugs and surgical thrombectomy, have been widely used, they often have limitations in terms of effectiveness, safety, and procedural time.
The current treatment landscape includes approved thrombectomy devices from various manufacturers, such as Medtronic, Stryker, and Penumbra. These devices offer improved clot retrieval capabilities, reduced procedure times, and enhanced patient outcomes compared to previous generations of thrombectomy devices.
However, there is still a significant need for more advanced and user-friendly thrombectomy devices that can further improve patient outcomes and procedural efficiency. Ongoing research and development efforts are focused on incorporating emerging technologies, such as robotics and artificial intelligence, into thrombectomy devices to enhance precision, reduce complications, and expand the applications of thrombectomy beyond acute ischemic stroke.
Some breakthrough treatment options currently being developed include novel thrombectomy devices with enhanced clot retrieval mechanisms, integration with advanced imaging and navigation technologies, and the use of combination therapies (e.g., mechanical thrombectomy combined with thrombolytic drugs).
The Thrombectomy Devices Market is relatively consolidated, with a few major players dominating the market. However, there is also a presence of specialized and innovative smaller companies, contributing to the overall competitive landscape and driving continued advancements in the field.
Market Report Scope:
|
Description |
|
|
The market size in 2023 |
US$ 1527.9 Mn |
|
CAGR (2024 - 2031) |
7.4% |
|
The revenue forecast in 2031 |
US$ 270270.8 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Medtronic plc, Stryker Corporation, Johnson & Johnson, Penumbra, Inc., Boston Scientific Corporation, Terumo Corporation, Acandis GmbH, Argon Medical Devices, Inc., AngioDynamics, Inc., Phenox GmbH, Neuravi Limited (acquired by Johnson & Johnson), Scitech Medical, Inc., Balt, Wallaby Medical, Imperative Care, Inc., Rapid Medical, Cerenovus (a Johnson & Johnson company), Merit Medical Systems, Inc., Perflow Medical, Silk Road Medical, Inc. |
Market Drivers:
Increasing Prevalence of Vascular Disorders
The growing incidence of vascular disorders, particularly acute ischemic stroke and deep vein thrombosis, is a significant driver for the Thrombectomy Devices Market. According to the World Stroke Organization, around 13.7 million new stroke cases are reported globally each year, and this number is expected to rise further in the coming years due to the aging population and unhealthy lifestyle factors. Similarly, the prevalence of deep vein thrombosis in the United States is estimated to be around 900,000 cases annually. The rising burden of these vascular conditions has increased the demand for effective and time-sensitive interventions, such as thrombectomy procedures, to restore blood flow and mitigate the risk of serious complications.
Advancements in Thrombectomy Device Technology
The Thrombectomy Devices Market has witnessed significant technological advancements in recent years, driving the adoption and efficacy of these devices. Manufacturers have been focused on developing more advanced, user-friendly, and minimally invasive thrombectomy devices to improve patient outcomes. For instance, the new generation of thrombectomy devices features enhanced clot retrieval mechanisms, reduced procedure times, and improved compatibility with advanced imaging and navigation technologies. These advancements have made thrombectomy procedures more effective, efficient, and accessible to a wider patient population, thereby fueling the growth of the market.
Growing Awareness of Minimally Invasive Procedures
There is an increasing awareness among healthcare providers and patients about the benefits of minimally invasive procedures, such as thrombectomy, compared to traditional surgical interventions. Thrombectomy devices offer a less invasive alternative to open surgical thrombectomy, resulting in reduced recovery times, lower risks of complications, and improved patient outcomes. As the awareness of these benefits spreads, the demand for thrombectomy devices is expected to rise, driving the growth of the market. Healthcare systems and policymakers are also actively promoting the adoption of minimally invasive treatments to improve patient outcomes and reduce the overall burden on healthcare resources.
Expanding Applications of Thrombectomy Devices
The Thrombectomy Devices Market is not limited to the treatment of acute ischemic stroke alone. Manufacturers are continuously exploring the expansion of thrombectomy device applications to address a wider range of vascular disorders, such as deep vein thrombosis, pulmonary embolism, and peripheral artery disease. As these new applications are approved and incorporated into clinical practice, the potential patient population for thrombectomy devices will grow, driving the overall market expansion. The versatility and adaptability of thrombectomy devices to address various vascular conditions are expected to be a key factor in the market's future growth.
Market Opportunities:
Technological Innovations and Integration
The Thrombectomy Devices Market presents significant opportunities for technological innovations and integration of advanced technologies, such as robotics and artificial intelligence (AI). Manufacturers are actively developing robotic-assisted thrombectomy systems that can enhance the precision and efficiency of clot removal procedures, leading to improved patient outcomes. Additionally, the integration of thrombectomy devices with advanced imaging and navigation technologies can provide healthcare providers with real-time, high-quality visualizations of the treatment area, enabling more informed decision-making and streamlined procedures. These technological advancements are expected to drive the adoption of thrombectomy devices and expand their applications beyond the current scope, opening up new avenues for market growth.
Expansion into Emerging Markets
The Thrombectomy Devices Market has significant growth potential in emerging markets, such as Asia-Pacific and Latin America, where the prevalence of vascular disorders is on the rise, but access to specialized stroke and vascular care is often limited. As these regions invest in improving their healthcare infrastructure and increasing the availability of advanced medical technologies, the demand for thrombectomy devices is anticipated to surge. Leading manufacturers can capitalize on this opportunity by establishing a strong presence in these emerging markets, tailoring their products to local needs, and collaborating with healthcare providers to increase awareness and adoption of thrombectomy procedures.
Reimbursement Initiatives and Regulatory Approvals
Favorable reimbursement policies and regulatory approvals can significantly drive the growth of the Thrombectomy Devices Market. Governments and healthcare authorities in various regions are recognizing the clinical benefits and cost-effectiveness of thrombectomy procedures, leading to the inclusion of thrombectomy devices in reimbursement schemes and the streamlining of regulatory approval processes. As more patients gain access to these life-saving treatments, the demand for thrombectomy devices will increase, presenting a lucrative opportunity for market expansion. Manufacturers can work closely with regulatory bodies and policymakers to navigate the approval and reimbursement landscape, further bolstering the growth of the Thrombectomy Devices Market.
Collaborations and Partnerships
Collaborations and partnerships between thrombectomy device manufacturers, healthcare providers, and research institutions can unlock significant growth opportunities in the market. These collaborations can focus on developing innovative thrombectomy solutions, conducting clinical studies to demonstrate the efficacy of these devices, and implementing educational programs to increase awareness among healthcare professionals and patients. By fostering these strategic alliances, manufacturers can leverage the expertise and resources of various stakeholders to drive innovation, improve patient outcomes, and expand the reach of thrombectomy devices, ultimately fueling the growth of the overall market.
Market Trends:
Adoption of Robotics and AI-Powered Technologies
The Thrombectomy Devices Market is witnessing a shift towards the adoption of robotic and AI-powered technologies to enhance the precision and efficacy of thrombectomy procedures. Manufacturers are developing innovative robotic-assisted thrombectomy systems that can improve operator control, reduce procedure times, and minimize the risk of complications. These advanced technologies not only enhance the clinical outcomes but also make thrombectomy procedures more accessible to a wider patient population. As healthcare providers become more familiar with these cutting-edge solutions and recognize their benefits, the adoption of robotic and AI-powered thrombectomy devices is expected to accelerate, driving the overall market trend.
Focus on User-Friendly and Versatile Devices
Manufacturers in the Thrombectomy Devices Market are increasingly focused on developing user-friendly and versatile devices that can cater to the needs of a diverse patient population and healthcare settings. This trend is driven by the desire to improve the overall experience for both patients and healthcare providers, leading to better clinical outcomes and higher adoption rates. The new generation of thrombectomy devices features enhanced ergonomics, improved compatibility with various imaging modalities, and the ability to address a wider range of vascular conditions, making them more appealing to healthcare providers and patients alike.
Expansion of Thrombectomy Applications beyond Stroke
While the primary application of thrombectomy devices has traditionally been the treatment of acute ischemic stroke, the market is witnessing a trend towards the expansion of these devices to address other vascular disorders, such as deep vein thrombosis, pulmonary embolism, and peripheral artery disease. Manufacturers are actively pursuing regulatory approvals and conducting clinical studies to demonstrate the efficacy of thrombectomy devices in treating a broader range of vascular conditions. As these new applications gain acceptance and become integrated into clinical practice, the potential patient population for thrombectomy devices will grow, driving the overall market trend.
Increasing Integration with Imaging and Navigation Technologies
Thrombectomy procedures require precise visualization of the treatment area to ensure successful clot removal and minimize the risk of complications. The Thrombectomy Devices Market is observing a trend towards the integration of these devices with advanced imaging and navigation technologies, such as CT angiography, MRI, and fluoroscopy. By seamlessly integrating thrombectomy devices with these imaging modalities, healthcare providers can obtain real-time, high-quality visualizations of the treatment area, leading to more informed decision-making and improved procedural outcomes. This integration of thrombectomy devices with cutting-edge imaging technologies is expected to continue gaining traction in the market.
Market Restraints:
High Cost of Thrombectomy Devices and Procedures
One of the primary restraints in the Thrombectomy Devices Market is the high cost associated with these devices and the corresponding procedures. Thrombectomy devices are sophisticated medical equipment that require significant research and development investments, leading to high production costs. Additionally, the specialized training and expertise required for healthcare providers to perform thrombectomy procedures can further contribute to the overall cost burden. This high cost can limit the accessibility of thrombectomy treatments, particularly in regions with limited healthcare budgets or inadequate reimbursement coverage. Addressing the affordability of thrombectomy devices and procedures will be crucial for expanding the market's reach and ensuring equitable access to these life-saving treatments.
Lack of Skilled and Trained Healthcare Professionals
The successful implementation of thrombectomy procedures requires a specialized set of skills and expertise from healthcare providers. The limited availability of trained and experienced interventional radiologists, neurologists, and vascular surgeons who can perform these complex procedures can act as a significant restraint to the growth of the Thrombectomy Devices Market. Healthcare systems in certain regions may struggle to keep up with the increasing demand for thrombectomy services, as the training and development of skilled professionals can be time-consuming and resource-intensive. Addressing this shortage of specialized healthcare providers through targeted training programs and the development of user-friendly thrombectomy devices will be essential for overcoming this market restraint.
Reimbursement Challenges in Certain Regions
Reimbursement policies and coverage for thrombectomy procedures can vary significantly across different regions and healthcare systems. In some markets, the lack of adequate reimbursement or the complex reimbursement landscape can hinder the adoption of thrombectomy devices, as healthcare providers and patients may be deterred by the out-of-pocket costs. This restraint is particularly prevalent in emerging markets or regions with underdeveloped healthcare infrastructure, where the inclusion of thrombectomy devices in reimbursement schemes may not be a priority. Manufacturers and policymakers need to collaborate to address these reimbursement challenges and ensure that thrombectomy treatments are accessible and affordable for patients in need.
Recent Developments:
|
Development |
Company Name |
|
In April 2023, Medtronic received FDA approval for its Solitaire X Revascularization Device, a next-generation thrombectomy device designed to improve clot retrieval and reduce procedure times for the treatment of acute ischemic stroke. |
Medtronic plc |
|
In September 2022, Penumbra, Inc. announced the launch of its new ACE68 aspiration thrombectomy system, which features enhanced aspiration power and a larger-diameter aspiration catheter for improved clot removal in ischemic stroke and other vascular occlusions. |
Penumbra, Inc. |
|
In June 2021, Stryker Corporation acquired Neurovasc Technologies, a company focused on developing innovative thrombectomy devices for the treatment of acute ischemic stroke. This acquisition strengthened Stryker's position in the thrombectomy devices market. |
Stryker Corporation |
|
In November 2020, Boston Scientific received FDA approval for its Penumbra ACE68 Reperfusion Catheter, a large-bore aspiration catheter designed to improve clot removal and recanalization in the treatment of acute ischemic stroke. |
Boston Scientific Corporation |
|
In March 2020, Terumo Corporation announced the launch of its Azur Peripheral Thrombectomy System, a mechanical thrombectomy device for the treatment of peripheral artery disease, expanding the company's thrombectomy portfolio beyond its stroke-focused products. |
Terumo Corporation |
These recent developments in the Thrombectomy Devices Market demonstrate the ongoing efforts by leading manufacturers to introduce advanced, user-friendly, and more effective thrombectomy devices to address the unmet needs in the treatment of vascular disorders, particularly acute ischemic stroke and peripheral artery disease.
Market Regional Insights:
The Thrombectomy Devices Market is a global market with significant regional variations in terms of market size, growth, and adoption patterns.
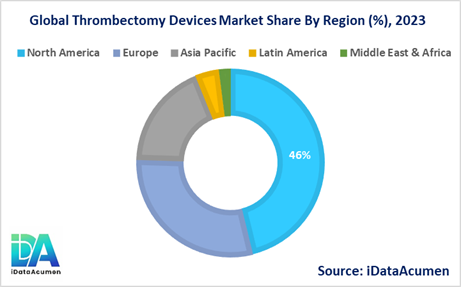
North America is expected to be the largest market for Thrombectomy Devices, accounting for over 46.2% of the market share in 2024. The growth of the market in North America is attributed to the high incidence of stroke and other vascular disorders, well-established healthcare infrastructure, and the presence of leading thrombectomy device manufacturers.
Europe is the second-largest market for Thrombectomy Devices, accounting for 29.3% of the market share in 2024. The growth of the market in Europe is driven by the increasing awareness of the benefits of thrombectomy procedures, improving access to specialized stroke and vascular care, and the adoption of advanced medical technologies.
The Asia Pacific region is expected to be the fastest-growing market for Thrombectomy Devices, with a CAGR of over 18.4% during the forecast period. The growth of the market in the Asia Pacific region is attributed to the rising incidence of stroke and other vascular disorders, increasing healthcare expenditure, and the expansion of healthcare infrastructure in developing countries like China and India.
The Latin America and Middle East & Africa regions account for smaller market shares, 4.1% and 2.0% respectively, in 2024. However, these regions are also expected to experience growth in the Thrombectomy Devices Market due to the increasing awareness of vascular disorders and the gradual improvements in healthcare access and infrastructure.
Market Segmentation:
- By Product Type
- Mechanical Thrombectomy Devices
- Aspiration Thrombectomy Devices
- Hybrid Thrombectomy Devices
- Rotational Thrombectomy Devices
- Others (including Ultrasonic Thrombectomy Devices, Chemical Thrombectomy Devices)
- By End-User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Others (including Diagnostic Centers, Research Institutions)
- By Application
- Acute Ischemic Stroke
- Deep Vein Thrombosis
- Pulmonary Embolism
- Peripheral Artery Disease
- Others (including Coronary Artery Disease, Renal Artery Thrombosis)
- By Technology
- Mechanical Thrombectomy
- Aspiration Thrombectomy
- Combination Thrombectomy
- Ultrasonic Thrombectomy
- Others (including Chemical Thrombectomy, Radiation-based Thrombectomy)
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Market Segment Analysis:
Among the various segments of the Thrombectomy Devices Market, the Mechanical Thrombectomy Devices segment is projected to be the largest and fastest-growing. This segment is expected to account for the largest market share in 2024 due to the superior clot retrieval capabilities, reduced procedure times, and improved patient outcomes associated with mechanical thrombectomy devices compared to traditional treatments.
The Mechanical Thrombectomy Devices segment is expected to grow at a CAGR of around 8.2% during the forecast period, driven by the increasing adoption of these devices in the treatment of acute ischemic stroke and other vascular disorders. The growth of this segment is particularly strong in North America and Europe, where there is a well-established network of specialized stroke centers and increased awareness of the benefits of mechanical thrombectomy.
Another rapidly growing segment is the Aspiration Thrombectomy Devices, which is expected to experience a CAGR of around 7.8% during the forecast period. The growth of this segment is attributed to the rising popularity of aspiration thrombectomy as a less invasive alternative to mechanical thrombectomy, with the potential for improved clot removal and reduced procedural complications.
The Asia Pacific region is anticipated to be the fastest-growing market for both the Mechanical Thrombectomy Devices and Aspiration Thrombectomy Devices segments, driven by the increasing incidence of stroke and other vascular disorders, improving healthcare infrastructure, and the expanding presence of leading thrombectomy device manufacturers in the region.
Top Companies in the Thrombectomy Devices Market:
- Medtronic plc
- Stryker Corporation
- Johnson & Johnson
- Penumbra, Inc.
- Boston Scientific Corporation
- Terumo Corporation
- Acandis GmbH
- Argon Medical Devices, Inc.
- AngioDynamics, Inc.
- Phenox GmbH
- Neuravi Limited (acquired by Johnson & Johnson)
- Scitech Medical, Inc.
- Balt
- Wallaby Medical
- Imperative Care, Inc.
- Rapid Medical
- Cerenovus (a Johnson & Johnson company)
- Merit Medical Systems, Inc.
- Perflow Medical
- Silk Road Medical, Inc.