Market Analysis:
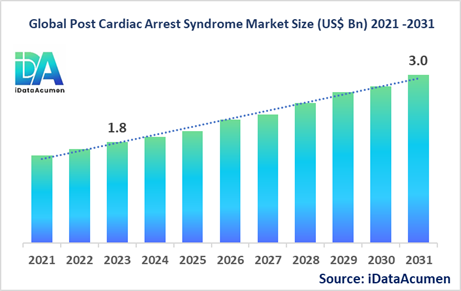
The Post Cardiac Arrest Syndrome Market size is expected to reach USD 3.0 billion by 2031, from USD 1.8 billion in 2023, at a CAGR of 6.7% during the forecast period. Post-cardiac arrest syndrome (PCAS) refers to a set of pathophysiological processes that occur after successful resuscitation from cardiac arrest. It is used to treat patients who have regained pulse after cardiac arrest to improve neurological and cardiovascular outcomes. The rising incidence of cardiovascular diseases and out of hospital cardiac arrests is driving demand for better post-resuscitation care.
The Post Cardiac Arrest Syndrome Market is segmented by product type, end-user, application, patient group and region. By product type, the therapeutic hypothermia systems segment accounted for the largest market share in 2023. The growth is attributed to the widespread use of therapeutic hypothermia in post-cardiac arrest care. For instance, Zoll Medical received FDA clearance in 2022 for its ThermoSuit System for managing body temperature in post-cardiac arrest patients.
Epidemiology Insights:
- Cardiac arrest has an annual incidence of around 300,000 to 350,000 cases in the US and 275,000 in Europe. Survival rates vary between 5-10% globally.
- Key factors driving epidemiological trends are rising prevalence of CVDs, growing geriatric population, and changes in lifestyle factors like smoking, obesity and physical inactivity.
- As per AHA estimates, the annual incidence of out-of-hospital cardiac arrest (OHCA) in the US was 356,500 in 2019 and the OHCA survival rate was 10.4%.
- In the EU, the reported OHCA incidence rate is 38 per 100,000 population. Incidence is higher in men than women.
- The increasing CVD incidence and cardiac arrest prevalence across mature and emerging markets is expected to drive growth opportunities for post-cardiac arrest treatments.
Market Landscape:
- There are high unmet needs for neurological and cardiovascular support in post-cardiac arrest care. Current standard treatments are limited to supportive critical care.
- Approved therapies include drugs like epinephrine, antiarrhythmics, vasopressors, and mechanical CPR devices. Therapeutic hypothermia is the only approved neuroprotective treatment.
- Several pharmaceuticals like cyclosporine, xenon gas, sulfide drugs are being evaluated as neuroprotective agents. Advanced extracorporeal life support devices are also being developed.
- Targeted temperature management, cerebral oximetry monitoring, neuroprotective drugs, stem cell therapy and ECMO are emerging breakthrough approaches.
- The market has mix of global medical device companies like Medtronic, Asahi Kasei, and pharmaceutical companies like BTG plc, Pfizer, Veloxis Pharmaceuticals.
Market Scope:
|
Key Insights |
Description |
|
The market size in 2023 |
US$ 1.8 Bn |
|
CAGR (2024 - 2031) |
6.7% |
|
The revenue forecast in 2031 |
US$ 3.0 Bn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2031 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Medtronic, Nihon Kohden, General Electric, Koninklijke Philips, Stryker, Asahi Kasei, Baxter International, Bardy Diagnostics, Zoll Medical, Physio-Control |
Market Drivers:
Rising Incidence of Cardiovascular Diseases
The rising prevalence of cardiovascular diseases (CVDs) such as coronary artery disease, arrhythmias, and hypertension is a major factor driving growth in the post-cardiac arrest syndrome market. CVDs are a leading cause of mortality globally and significantly increase the risk of sudden cardiac arrest. According to research, over 80% of out-of-hospital cardiac arrest cases occur due to underlying CVDs. As the incidence of CVDs increases due to sedentary lifestyles, obesity and demographic shifts, the patient pool requiring post-resuscitation care and management of post-cardiac arrest syndrome will also expand. This presents significant opportunities for market growth.
Advancements in Emergency Medical Services
Advancements in emergency medical services (EMS) and wider availability of AEDs has improved out-of-hospital cardiac arrest survival rates in recent years. Research indicates that early CPR and defibrillation within 3-5 minutes of collapse can increase survival odds by 2 to 4 times. Authorities across mature and emerging markets are focused on strengthening EMS infrastructure and capabilities. EMS providers are also utilizing technologies like mobile integrated healthcare and telemedicine to improve post-arrest care during patient transport to hospitals. The progress in EMS is leading to more patients being resuscitated successfully after cardiac arrest and requiring post-resuscitation therapies.
Increasing Industry Focus on Developing Novel Therapies
Several pharmaceutical and medical device companies are channelizing R&D efforts to develop innovative therapies and technologies for post-cardiac arrest syndrome management. Research is ongoing for neuroprotective drugs, advanced extracorporeal support devices, cerebral oximetry for brain monitoring, targeted temperature management systems, biomarkers and precision medicine approaches. Product approvals and launches like Zoll Medical’s ThermoSuit system, BardyDx’s Carnation Ambulatory Monitor, and advances in extracorporeal membrane oxygenation will positively impact market growth.
Expanding Government Initiatives to Strengthen Emergency Care
Expanding government investments to improve emergency care infrastructure and services is contributing to increased adoption of post-cardiac arrest treatments. For instance, the Cardiac Arrest Survival Act in the US is aimed at improving funding and coordination for cardiac arrest research. The Stopcardiacarrest initiative by the European Patient’s Forum is working to raise public awareness about the use of AEDs. Such programs are being supported by favorable policies to strengthen bystander response and EMS capabilities. This is creating a conducive environment for growth of the post-cardiac arrest syndrome market.
Market Opportunities:
Growing Opportunity in Developing Countries
Developing countries represent a significant opportunity for growth of the post-cardiac arrest syndrome market owing to improving healthcare infrastructure, rising healthcare expenditure and increasing CVD incidence. According to the WHO, around 80% of CVD deaths occur in low and middle-income countries. As disposable incomes rise and governments focus on improving emergency care capabilities, adoption of advanced resuscitation devices and post-arrest treatments will increase. Market players can focus on improving accessibility and affordability to tap into the large underpenetrated patient pool.
Rising Potential for Neuroprotective Drugs
Neuroprotective drugs to mitigate brain injury post-cardiac arrest hold significant potential. R&D is ongoing to develop novel pharmaceuticals that can be administered during or after resuscitation to improve neurological outcomes. Drugs like cyclosporine, erythropoietin, xenon and hydrogen sulfide are being evaluated in clinical trials. Positive research outcomes and regulatory approvals will create new growth avenues. Moreover, synergistic therapies combining neuroprotective drugs with hypothermia also hold promise. Market players can focus on drug pipeline development in this area.
Innovations in Post-Hospitalization Care
Increasing survival post-discharge after cardiac arrest represents an opportunity for market players to improve post-hospitalization care for better long-term outcomes. Areas like cardiac rehabilitation, home-based nursing, remote monitoring through mHealth devices, and counselling hold potential for enhancing recovery and minimizing risks. Private players can collaborate with hospitals and rehabilitation centers to offer bundled post-discharge services. Insurers can also include post-cardiac arrest syndrome therapies in their coverage.
Widening Applications of Extracorporeal Life Support
The applications of extracorporeal life support (ECLS) technologies like extracorporeal cardiopulmonary resuscitation (ECPR) and extracorporeal membrane oxygenation (ECMO) are widening for cardiovascular stabilization post-cardiac arrest. Ongoing advances to make these technologies more compact, portable and patient-friendly will support adoption. Increased research validating ECMO’s role in improving 60-90 day survival compared to standard CPR, presents opportunities for market players. Incorporating ECMO training in EMS and emergency departments can open up growth avenues.
Market Trends:
Emergence of Targeted Temperature Management
Targeted temperature management (TTM) is an emerging trend where advanced systems enable meticulous temperature control based on patient-specific parameters. Initial hypothermia is followed by controlled rewarming matched to the patient’s hemodynamics, oxygenation status etc. Players like Asahi Kasei, Bard Medical and Zoll Medical offer TTM systems. Research indicates TTM is more effective than conventional therapeutic hypothermia. The approach holds significance for individualized post-cardiac arrest treatment.
Shifting Focus Towards Cerebral Oximetry
Providers are increasingly using cerebral oximetry monitoring during post-cardiac arrest treatment to detect ischemic changes in brain oxygen saturation. Several studies have validated that maintaining cerebral oxygen saturation within target range in the first 24 hours is associated with improved neurological outcomes. Players like Medtronic, Nonin Medical and Masimo offer cerebral oximetry systems specially designed for cardiac arrest patients. Their integration with therapeutic hypothermia is gaining traction.
Emergence of Wearable Defibrillators
The advent of wearable cardioverter defibrillator (WCD) therapy enables constant monitoring and delivery of shock during potentially fatal arrhythmias in post discharge patients. External devices like Zoll Medical’s LifeVest allow stabilization until the patient regains heart function. Research indicates WCDs improve survival in post-arrest patients during the high-risk phase. Their role as a bridge to ICD implant is driving adoption. Furthermore, development of leadless, ECG-based WCDs will support growth.
Progress in Biomarker Research
Research on biomarkers to predict outcomes post-cardiac arrest is gaining momentum. Trials are analyzing the usefulness of serum biomarkers like serum tau, S100 calcium-binding protein B and creatine kinase in determining neurological prognosis. Other markers being studied include neuroimaging, electroencephalography etc. The development of biomarker-based prediction models holds potential to optimize post-cardiac arrest treatment decisions and improve standardization.
Market Restraints:
Limited Awareness among General Population
Lack of awareness among the general public about sudden cardiac arrest and optimal post-resuscitation care is a major restrain for the market. Low familiarity with life-saving interventions like CPR and defibrillation hampers timely emergency response. Delays in CPR negatively impact survival and neurological outcomes. Moreover, limited understanding of post-cardiac arrest syndrome worsens prognosis. Raising community awareness through public engagement initiatives is essential.
High Costs and Reimbursement Challenges
The costs associated with post-cardiac arrest treatments like therapeutic hypothermia, advanced monitoring and extracorporeal life support are usually high. At the same time, reimbursement coverage challenges and budget constraints in healthcare systems limit adoption. For instance, reimbursement for ECMO therapy is still variable across different regions. Reducing costs through technological improvements and increasing insurance coverage can help overcome affordability issues.
Paucity of Robust Clinical Evidence
There is a lack of large-scale, concrete clinical evidence elucidating the long-term benefits of emerging therapies like ECMO, neuroprotective drugs etc. for post-cardiac arrest syndrome. Variable results from different trials lead to uncertainties in treatment guidelines. Generating more high-quality evidence through rigorous, coordinated research is vital to validate efficacy and support widespread adoption. Standardization of protocols also needs to be improved significantly.
Recent Developments:
|
Development |
Involved Company |
|
FDA approval for Carnation Ambulatory Monitor |
Bardy Diagnostics |
|
Launch of Arctic Sun Stat temperature management |
Bard Medical |
|
Acquisition of Thermacor temperature management business |
Asahi Kasei Corporation |
|
Product Launch |
Company Name |
|
In Jan 2022, Zoll received FDA clearance for its ThermoSuit System for temperature management in post-cardiac arrest patients. It provides efficient cooling and precision temperature control. |
Zoll Medical |
|
In March 2021, BardyDx received FDA approval for Carnation Ambulatory Monitor (CAM), a patch-based ambulatory cardiac monitor to detect arrhythmias in post-cardiac arrest patients. |
Bardy Diagnostics |
Market Regional Insights:
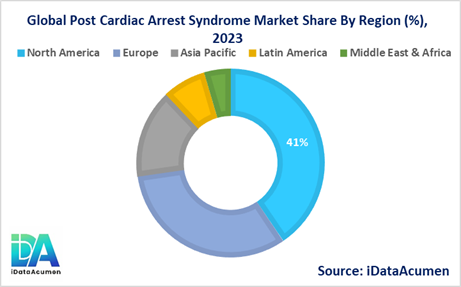
- North America is expected to be the largest market for Post Cardiac Arrest Syndrome Market during the forecast period, accounting for over 40% of the market share in 2023. The growth is driven by high incidence of cardiac arrests, established healthcare infrastructure and reimbursement coverage.
- Europe market is expected to be the second-largest market, accounting for over 32% market share in 2023. The growth is driven by rising prevalence of CVDs and government funding for emergency care services.
- Asia Pacific market is expected to be the fastest-growing market with a CAGR of 9% during forecast period owing to improving healthcare infrastructure, growing medical tourism and increasing focus of global companies in these markets.
Market Segmentation:
- By Product Type
- Therapeutic Hypothermia Systems
- Monitoring Devices
- Advanced Resuscitation Devices
- Diagnostic Equipment
- Medications
- By End-User
- Hospitals
- Emergency Medical Services
- Ambulatory Surgical Centers
- Trauma Centers
- Long Term Care Centers
- By Application
- Ventricular Fibrillation
- Asystole
- Pulseless Electrical Activity
- Others
- By Patient Group
- Adults
- Pediatric
- Geriatric
- By Regions
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Top companies in the Post Cardiac Arrest Syndrome Market:
- Medtronic
- Nihon Kohden
- General Electric
- Koninklijke Philips
- Stryker
- Asahi Kasei
- Baxter International
- Bardy Diagnostics
- BIOTRONIK
- Physio-Control
- Abbott Laboratories
- Boston Scientific
- Cardiac Science
- Livanova
- Midmark
- Mindray Medical
- Schiller
- Shenzhen Mindray Bio-Medical Electronics
- Zoll Medical
- Positech Healthcare