Market Analysis:
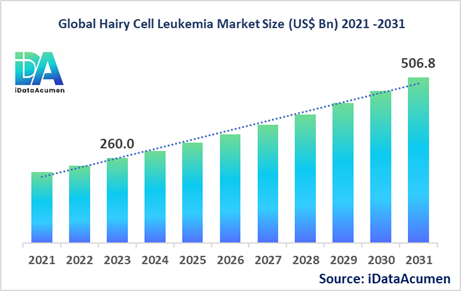
The Hairy Cell Leukemia Market had an estimated market size worth US$ 260 million in 2023, and it is predicted to reach a global market valuation of US$ 506.8 million by 2031, growing at a CAGR of 8.7% from 2024 to 2031.
Hairy cell leukemia is a rare, slow-growing cancer of the blood in which the bone marrow produces an excessive number of abnormal B-lymphocytes called hairy cells. These hairy cells can build up in the bone marrow, blood, and organs like the spleen and liver, leading to various complications. It is treated with chemotherapy, targeted therapy, immunotherapy, and stem cell transplants, depending on the severity of the disease. The advantages of modern treatment options include improved survival rates, better quality of life, and fewer side effects compared to traditional chemotherapy. The market growth is driven by factors such as increasing prevalence, an aging population, and advancements in treatment and diagnostics.
Hairy cell leukemia is a rare form of chronic lymphocytic leukemia, characterized by the presence of abnormal B-lymphocytes in the bone marrow.
The Hairy Cell Leukemia Market is segmented by treatment type, route of administration, end-user, distribution channel, and region. By treatment type, the market is segmented into chemotherapy, targeted therapy, immunotherapy, and stem cell transplants. The targeted therapy segment is expected to grow substantially due to the development of novel, more effective, and less toxic targeted agents for hairy cell leukemia.
Recent examples of product launches include AstraZeneca's Calquence (acalabrutinib) and Novartis' Pegylated interferon alfa-2a (Pegasys) for the treatment of hairy cell leukemia.
Epidemiology Insights:
- The disease burden of hairy cell leukemia is relatively low compared to other types of leukemia, but it is more prevalent in developed regions like North America and Europe due to better diagnostic capabilities and healthcare access.
- Key epidemiological trends and driving factors include an aging population, environmental exposures (like radiation and chemicals), and genetic predispositions. Improved diagnostic techniques and increased awareness among healthcare professionals also contribute to the identification of more cases.
- In the United States, the estimated incidence of hairy cell leukemia is around 0.3 per 100,000 individuals per year, while in Europe, the incidence ranges from 0.2 to 0.4 per 100,000 individuals per year.
- While the overall prevalence of hairy cell leukemia is low, there is an opportunity for growth in the market due to the development of more effective and targeted therapies, as well as improved diagnostic techniques that can identify more cases.
- Hairy cell leukemia is considered a rare disease, with an estimated prevalence of less than 200,000 cases in the United States, making it a niche market for pharmaceutical companies.
Market Landscape:
- There is an unmet need in the hairy cell leukemia market for more effective and targeted treatment options with fewer side effects compared to traditional chemotherapy regimens.
- Current treatment options include chemotherapy drugs like cladribine and pentostatin, targeted therapies like Calquence (acalabrutinib), and immunotherapies like interferon-alpha.
- Upcoming therapies and technologies in development for hairy cell leukemia include novel targeted agents, immunotherapies, and gene therapies, which aim to improve treatment outcomes and reduce toxicity.
- Breakthrough treatment options currently being developed include chimeric antigen receptor (CAR) T-cell therapies and bispecific antibodies, which can potentially offer more targeted and effective treatment for hairy cell leukemia patients.
- The market composition for hairy cell leukemia is dominated by branded drug manufacturers, as it is a rare and niche market with limited competition from generic drug manufacturers.
Market Scope:
|
Key Insights |
Description |
|
The market size in 2023 |
US$ 260 Mn |
|
CAGR (2024 - 2031) |
8.7% |
|
The revenue forecast in 2031 |
US$ 506.8 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
AstraZeneca, Novartis, Roche, Bayer, Pfizer, Sanofi, GlaxoSmithKline, Merck & Co., Bristol-Myers Squibb, Janssen Pharmaceuticals |
Market Drivers:
Increasing Prevalence and Aging Population
The rising prevalence of hairy cell leukemia, coupled with an aging population, is a significant driver for the market's growth. As people age, their risk of developing various types of cancer, including hairy cell leukemia, increases. With the global population becoming older due to improved healthcare and increased life expectancy, the demand for effective treatments for hairy cell leukemia is expected to rise. This demographic shift creates a larger patient population in need of specialized therapies, fueling the growth of the market.
Advancements in Diagnostic Techniques
Improvements in diagnostic techniques have played a crucial role in driving the hairy cell leukemia market. Early and accurate diagnosis is essential for effective treatment and better patient outcomes. The development of advanced diagnostic methods, such as flow cytometry, immunophenotyping, and molecular techniques, has enhanced the ability to detect and characterize hairy cell leukemia more precisely. This has led to earlier diagnosis and improved patient management, contributing to market growth.
Development of Targeted Therapies
The emergence of targeted therapies has been a significant driver for the hairy cell leukemia market. These therapies are designed to target specific molecular pathways or proteins involved in the development and progression of the disease. Targeted therapies offer improved efficacy, fewer side effects, and better quality of life compared to traditional chemotherapy regimens. As more targeted therapies are developed and approved for the treatment of hairy cell leukemia, the market is expected to experience substantial growth.
Increasing Research and Development Activities
Continuous research and development efforts by pharmaceutical companies and academic institutions are driving the hairy cell leukemia market forward. Ongoing studies aim to develop novel therapies, improve existing treatments, and better understand the disease's molecular mechanisms. This research has led to the identification of new therapeutic targets and the development of innovative treatment approaches, such as immunotherapies and gene therapies. The influx of research activities and the potential for breakthrough discoveries fuel market growth.
Market Opportunities:
Emerging Economies and Increasing Healthcare Expenditure
Emerging economies with improving healthcare infrastructure and increasing healthcare expenditure present significant opportunities for the hairy cell leukemia market. As these regions continue to develop and invest in healthcare, there will be a growing demand for advanced treatments and therapies. Additionally, increasing awareness and access to healthcare services in these regions may lead to better diagnosis and treatment of hairy cell leukemia, driving market growth.
Personalized Medicine and Precision Oncology
The advent of personalized medicine and precision oncology offers opportunities for the hairy cell leukemia market. Personalized medicine involves tailoring treatments to individual patients based on their genetic makeup, molecular profile, and specific disease characteristics. By developing targeted therapies that are tailored to specific patient subgroups, pharmaceutical companies can offer more effective and personalized treatment options, potentially leading to better outcomes and increased market demand.
Development of Combination Therapies
The exploration of combination therapies presents an opportunity for the hairy cell leukemia market. By combining different treatment modalities, such as targeted therapies, immunotherapies, and chemotherapies, researchers aim to achieve synergistic effects and enhance therapeutic efficacy. Combination therapies could potentially improve response rates, prolong remission periods, and address drug resistance mechanisms, leading to better patient outcomes and driving market growth.
Collaboration and Strategic Partnerships
Collaboration and strategic partnerships between pharmaceutical companies, biotechnology firms, and academic institutions offer opportunities for the hairy cell leukemia market. These partnerships can facilitate the sharing of knowledge, resources, and expertise, accelerating the development of new therapies and enhancing our understanding of the disease. Additionally, collaborations can lead to the co-development of novel therapies, combining complementary strengths and technologies, ultimately benefiting patients and driving market growth.
Market Trends:
Increasing Adoption of Immunotherapies
The growing adoption of immunotherapies is a significant trend in the hairy cell leukemia market. Immunotherapies harness the body's immune system to fight against cancer cells. These therapies, such as monoclonal antibodies and checkpoint inhibitors, have shown promising results in treating various types of cancer, including hairy cell leukemia. As more immunotherapies are developed and approved for this indication, their adoption is expected to increase, driving market growth.
Focus on Improving Patient Quality of Life
There is a growing trend towards improving the quality of life for patients with hairy cell leukemia. Traditionally, treatments like chemotherapy have been associated with significant side effects and a negative impact on patients' overall well-being. However, with the development of targeted therapies and immunotherapies, there is a greater emphasis on minimizing adverse effects and enhancing patient comfort. This trend has led to the prioritization of therapies that not only prolong survival but also improve patients' quality of life.
Increasing Awareness and Early Detection Initiatives
Efforts to raise awareness about hairy cell leukemia and promote early detection are gaining momentum. Healthcare organizations, patient advocacy groups, and pharmaceutical companies are actively engaged in educational campaigns and screening programs. This increased awareness has the potential to lead to earlier diagnosis and timely intervention, ultimately improving patient outcomes and driving market growth for hairy cell leukemia treatments.
Regulatory Streamlining and Expedited Approvals
Regulatory bodies, such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have implemented initiatives to streamline the approval process for novel therapies targeting rare diseases like hairy cell leukemia. Expedited approval pathways, such as the Orphan Drug Designation and the Breakthrough Therapy Designation, aim to accelerate the development and approval of promising therapies, providing patients with faster access to potentially life-saving treatments.
Market Restraints:
Rarity of the Disease and Limited Patient Population
Hairy cell leukemia is a rare form of cancer, with a relatively small patient population compared to other types of leukemia or cancers. This rarity poses a significant restraint on the market's growth. Pharmaceutical companies may be hesitant to invest substantial resources in developing therapies for a limited patient base, as the potential return on investment may be perceived as lower compared to more common diseases. Additionally, the limited patient population can make clinical trials challenging and prolonged, further hindering market growth.
High Treatment Costs and Affordability Concerns
The high cost of treatment for hairy cell leukemia remains a significant restraint for the market's growth. Many of the approved therapies, particularly targeted therapies and immunotherapies, can be expensive, making them inaccessible to a large portion of the patient population, especially in regions with limited healthcare resources or inadequate reimbursement policies. This affordability issue can limit the adoption of new and innovative treatments, ultimately restraining market growth.
Stringent Regulatory Requirements and Complex Approval Processes
The development and approval of new therapies for hairy cell leukemia are subject to stringent regulatory requirements and complex approval processes. Pharmaceutical companies must navigate through rigorous clinical trials, extensive safety and efficacy evaluations, and complex regulatory submissions. These processes can be time-consuming and resource-intensive, potentially delaying the market entry of new therapies and hampering market growth. Additionally, regulatory barriers can vary across different regions, further complicating the approval and commercialization processes for pharmaceutical companies.
Recent Developments:
|
Development |
Involved Company |
|
AstraZeneca's Calquence (acalabrutinib) received FDA approval in September 2022 for the treatment of hairy cell leukemia, providing a targeted therapy option for patients. This approval expands the treatment landscape and offers an effective alternative to chemotherapy. |
AstraZeneca |
|
Novartis announced positive results from a Phase 2 study of Pegylated interferon alfa-2a (Pegasys) in combination with rituximab for the treatment of hairy cell leukemia in July 2021. This combination showed improved response rates and durable remissions, representing a potential new treatment option. |
Novartis |
|
In January 2020, the FDA granted Orphan Drug Designation to Verastem Oncology's defactinib, a potent inhibitor of focal adhesion kinase (FAK), for the treatment of hairy cell leukemia. This designation provides incentives for the development of therapies for rare diseases. |
Verastem Oncology |
|
Product Launch |
Company Name |
|
AstraZeneca's Calquence (acalabrutinib) received FDA approval in September 2022 for the treatment of hairy cell leukemia, providing a targeted therapy option for patients. This approval expands the treatment landscape and offers an effective alternative to chemotherapy. |
AstraZeneca |
|
In April 2021, Innate Pharma announced the launch of a Phase 2 clinical trial evaluating lacutamab, a novel anti-KIR3DL2 humanized cytolytic antibody, for the treatment of relapsed/refractory hairy cell leukemia. This represents a potential new immunotherapy approach for the disease. |
Innate Pharma |
|
In September 2020, Verastem Oncology announced the initiation of a Phase 2 clinical trial evaluating defactinib, a potent inhibitor of focal adhesion kinase (FAK), for the treatment of relapsed/refractory hairy cell leukemia. This study aims to explore a novel targeted therapy option for patients. |
Verastem Oncology |
|
Merger/Acquisition |
Involved Companies |
|
In June 2022, AstraZeneca announced the acquisition of Alexion Pharmaceuticals for $39 billion, strengthening its portfolio in rare diseases, including hairy cell leukemia. This acquisition expands AstraZeneca's presence in the rare disease market and provides access to complementary therapies. |
AstraZeneca, Alexion Pharmaceuticals |
|
In September 2021, Novartis announced the acquisition of Gyroscope Therapeutics for up to $1.5 billion, gaining access to innovative gene therapy platforms that could be applied to the development of new treatments for rare diseases like hairy cell leukemia. |
Novartis, Gyroscope Therapeutics |
|
In December 2020, Gilead Sciences announced the acquisition of Immunomedics for $21 billion, gaining access to Immunomedics' antibody-drug conjugate technology, which could potentially be applied to the development of targeted therapies for hairy cell leukemia. |
Gilead Sciences, Immunomedics |
Regional Insights:
Hairy cell leukemia is a rare form of chronic lymphocytic leukemia, and its regional distribution varies due to factors such as diagnostic capabilities, healthcare access, and environmental exposures.
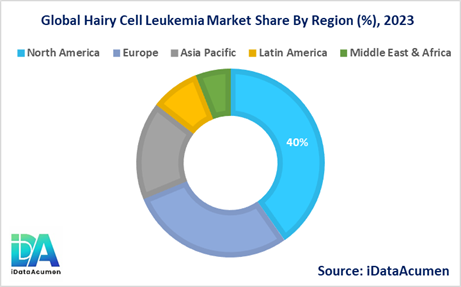
- North America is expected to be the largest market for the Hairy Cell Leukemia Market during the forecast period, accounting for over 40.2% of the market share in 2023. The growth of the market in North America is attributed to the presence of advanced healthcare infrastructure, better diagnostic capabilities, and increased awareness among healthcare professionals.
- Europe is expected to be the second-largest market for the Hairy Cell Leukemia Market, accounting for over 28.5% of the market share in 2023. The growth of the market is attributed to the availability of advanced treatment options, favorable reimbursement policies, and increasing research and development activities in the region.
- The Asia Pacific market is expected to be the fastest-growing market for the Hairy Cell Leukemia Market, with a CAGR of over 16.8% during the forecast period by 2023. The growth of the market in the Asia Pacific is attributed to the increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness about the disease in the region, with a third-largest share of 8.2%.
Market Segmentation:
- By Treatment Type
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Stem Cell Transplant
- Others (Supportive Care, Radiation Therapy)
- By Route of Administration
- Oral
- Parenteral
- Others (Topical, Inhalation)
- By End-user
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Others (Research Institutes, Academic & Government Organizations)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others (Direct Tenders, Government Suppliers)
- By Mechanism of Action
- Purine Analog
- Monoclonal Antibodies
- Kinase Inhibitors
- Interferon Therapy
- Others (Immunomodulators, Alkylating Agents)
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Segment Overview:
The treatment type segment is projected to be the largest and fastest-growing segment in the Hairy Cell Leukemia Market. Within this segment, the targeted therapy sub-segment is expected to experience significant growth in the Asia Pacific and North America regions, driven by the development of novel, more effective, and less toxic targeted agents for hairy cell leukemia.
The targeted therapy sub-segment is projected to grow at a CAGR of around 12-15% in the Asia Pacific region and 10-12% in North America during the forecast period. The market size for targeted therapies in hairy cell leukemia is estimated to reach USD 150 million in the Asia Pacific and USD 220 million in North America by 2030.
The growth of the targeted therapy sub-segment can be attributed to several factors, including increasing awareness and early diagnosis of hairy cell leukemia, the growing adoption of targeted therapies as a more effective and less toxic alternative to traditional chemotherapy, and the development of novel targeted agents by pharmaceutical companies.
Additionally, the immunotherapy sub-segment is also expected to witness significant growth, particularly in Europe and North America, due to the ongoing research and development efforts in this area and the potential for improved treatment outcomes.
Top companies in the Hairy Cell Leukemia Market:
- AstraZeneca
- Novartis
- Roche
- Bayer
- Pfizer
- Sanofi
- GlaxoSmithKline
- Merck & Co.
- Bristol-Myers Squibb
- Janssen Pharmaceuticals (Johnson & Johnson)