Companion diagnostics (CDx) is a term generally applied to an in-vitro diagnostic device. Companion diagnostics test is used to identify if a particular drug or therapy is safe and effective for a patient. Companion diagnostics is a litmus test for the corresponding drug or therapy. It helps to determine effectiveness of the drug outweighs its side effects. CDx can help to determine;
- Patients who are mostly to be benefitted by from a particular therapy;
- Patients who are likely to have serious side effects from a particular therapy;
- Monitoring response of a ongoing treatment to adjust the dosing to achieve improved effectiveness
Market Drivers and Restraints:
- Increasing amount of regulatory approvals, and product launches of targeted therapies is expected to support global companion diagnostics market growth over the forecast period. For instance, in September 2022, Thermo Fisher Scientific announced that its Oncomine Dx Target Test has received approval as a companion diagnostic (CDx) from the U.S. Food and Drug Administration (FDA).
- Increasing prevalence of diseases such as cancer is expected to support global companion diagnostics market growth over the forecast period. For instance, according to World Cancer Research Fund (WCRF), there were ~18.1 million cancer cases globally in 2020.
However, there are certain limitations associated with companion diagnostics such as low awareness, low availability in developing economies, inconsistent, and unreliable test results. These factors are expected to restrain global companion diagnostics market growth up to certain extent.
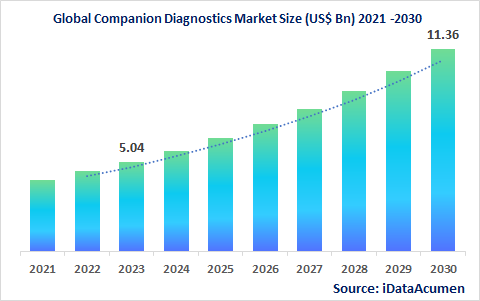
Global Companion Diagnostics Market accounted for US$ 4.0 Bn in 2021 and is expected to witness a CAGR of 12.3% over the forecast period (2022-2030) to reach US$ 11.3 Bn by 2030
Competitive Landscape:
The global companion diagnostics market is driven by technological advancement, and research and development. Multinational corporations and smaller companies operating in the industry are developing advanced personalized companion tests for available targeted therapies.
We have employed tools and techniques such as PEST analysis, SWOT analysis, PORTER’s analysis, BCG matrix to analyze and provide competitive and market intelligence.
Some of the prominent players operating in the market are:
- F. Hoffmann-La Roche Ltd.
- Qiagen N.V.
- GE Healthcare
- Thermo Fischer Scientific
- Agilent Technologies, Inc.
- Abbott Laboratories
- Guardant Health, Inc.
- Dako Denmark A/S
- Ventana Medical Systems, Inc.
- Biogenex Laboratories, Inc.
- Myriad Genetic Laboratories, Inc.
Segments Covered in the Global Companion Diagnostics Market Report:
The global companion diagnostics market is segmented based on product type, technology, disease indication, and region as follows:
- Product Type (Revenue, USD Billion; 2022–2030)
- Diagnostics Kits
- Assays
- Others
- Technology (Revenue, USD Billion; 2022–2030)
- PCR
- NGS
- ISH
- IHC
- Disease Indication (Revenue, USD Billion; 2022–2030)
- Breast Cancer
- Lung Cancer
- Blood Cancer
- Colorectal Cancer
- Others
- Region (Revenue, USD Billion; 2022–2030)
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- ASEAN
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of LATAM
- Middle East & Africa
- Saudi Arabia
- UAE
- Rest of MEA
- North America