Market Analysis:
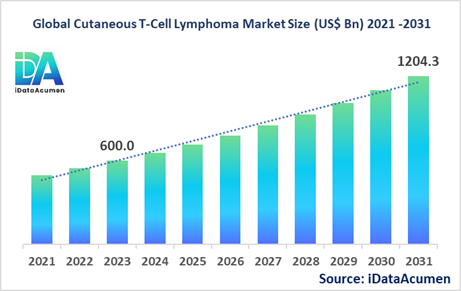
The Cutaneous T-Cell Lymphoma (CTCL) market had an estimated market size worth US$ 600 million in 2023, and it is predicted to reach a global market valuation of US$ 1,204.3 million by 2031, growing at a CAGR of 9.1% from 2024 to 2031.
CTCL is a rare type of non-Hodgkin lymphoma, a cancer of the lymphatic system that primarily affects the skin. In CTCL, abnormal T-cells (a type of white blood cell) accumulate in the skin, causing various skin lesions, rashes, and tumors. The disease can progress and spread to other organs if left untreated.
The key drivers of the CTCL market include the increasing incidence and prevalence of the disease, advancements in diagnostic techniques, the growing adoption of targeted therapies and immunotherapies, and favorable reimbursement policies for CTCL treatments.
The Cutaneous T-Cell Lymphoma Market is segmented by product type, disease type, end-user, distribution channel, and route of administration. The topical therapies segment is one of the largest subsegments, as these treatments are often the first-line approach for managing early-stage CTCL. The recent launch of Poteligeo (mogamulizumab) by Kyowa Kirin, a monoclonal antibody approved for the treatment of CTCL, has significantly contributed to the growth of the CTCL market.
Epidemiological Insights:
Cutaneous T-Cell Lymphoma is a rare disease, with a global incidence estimated to be around 3.9 per 100,000 individuals. The disease burden is highest in North America and Europe, with the United States and European Union accounting for the majority of CTCL cases worldwide.
The key epidemiological trends driving the CTCL market include an aging population, increased awareness and early diagnosis, and the rising prevalence of underlying conditions that can increase the risk of CTCL, such as HIV/AIDS and other immunodeficiency disorders. The growing patient population, particularly in developing regions, presents significant growth opportunities for the CTCL market.
Market Landscape:
The CTCL market has several unmet needs, particularly related to the limited availability of effective and well-tolerated treatment options. The current treatment landscape includes topical therapies, systemic therapies, phototherapy, and combination approaches. While these treatments can provide symptomatic relief and temporary remission, many patients eventually experience disease progression or relapse.
Upcoming therapies and technologies in the CTCL pipeline include targeted therapies, such as small molecule inhibitors and monoclonal antibodies, as well as novel immunotherapies. These breakthrough treatments are aimed at improving clinical outcomes, reducing side effects, and providing more durable responses for CTCL patients.
The CTCL market is moderately consolidated, with a few large pharmaceutical companies, such as Kyowa Kirin, Miragen Therapeutics, and Actelion Pharmaceuticals (a Janssen Pharmaceutical Company), dominating the landscape. However, there is also a presence of smaller, specialized biotech and research-oriented companies actively contributing to the development of innovative CTCL therapies.
Market Report Scope:
|
Description |
|
|
The market size in 2023 |
US$ 600 Mn |
|
CAGR (2024 - 2031) |
9.1% |
|
The revenue forecast in 2031 |
US$ 1,204.3 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Genzyme Corporation, Sanofi S.A, Cadila Healthcare, Mylan N.V., IDsA Generics, Cipla Ltd., Sun Pharmaceutical, Dr. Reddy’s Laboratories Ltd., Accord Healthcare, Natco Pharma, Biocon Ltd., Intas Pharmaceuticals, Pfizer Inc. |
Market Drivers:
Rising Incidence and Prevalence of Cutaneous T-Cell Lymphoma
The increasing incidence and prevalence of Cutaneous T-Cell Lymphoma (CTCL) is a significant driver fueling the growth of the CTCL market. CTCL is a rare type of non-Hodgkin lymphoma, and its incidence has been steadily rising over the past few years. According to recent epidemiological studies, the global incidence of CTCL is estimated to be around 3.9 per 100,000 individuals, with a higher prevalence in North America and Europe. The aging population, environmental factors, and the rising prevalence of underlying conditions like HIV/AIDS and other immunodeficiency disorders have all contributed to the increasing number of CTCL cases worldwide. As the patient population continues to grow, the demand for effective and innovative CTCL treatments has also escalated, driving the expansion of the CTCL market.
Advancements in Diagnostic Techniques
The advancements in diagnostic techniques for Cutaneous T-Cell Lymphoma have been a crucial driver for the growth of the CTCL market. Improved diagnostic methods, such as immunohistochemistry, flow cytometry, and molecular genetic analysis, have enabled earlier and more accurate detection of CTCL. These advanced diagnostic tools have allowed healthcare providers to identify the disease in its early stages, leading to timely intervention and improved patient outcomes. Early diagnosis is particularly important in CTCL, as the disease can progress and spread to other organs if left untreated. The availability of these advanced diagnostic techniques has not only enhanced the understanding of CTCL but has also paved the way for the development of targeted therapies and personalized treatment approaches, driving the growth of the CTCL market.
Increasing Adoption of Targeted Therapies and Immunotherapies
The growing adoption of targeted therapies and immunotherapies for the treatment of Cutaneous T-Cell Lymphoma has been a significant driver for the CTCL market. Targeted therapies, such as monoclonal antibodies and small molecule inhibitors, have demonstrated improved efficacy and better tolerability compared to traditional chemotherapy and radiation treatments. These targeted approaches aim to selectively disrupt the signaling pathways and mechanisms that are crucial for the survival and proliferation of CTCL cells, leading to more effective disease management and reduced side effects. Similarly, the emergence of immunotherapies, which harness the body's immune system to fight cancer, has also contributed to the growth of the CTCL market. Immunotherapeutic agents, such as checkpoint inhibitors and cytokine-based therapies, have shown promising results in the treatment of CTCL, providing new treatment options for patients and fueling the expansion of the CTCL market.
Favorable Reimbursement Policies
Favorable reimbursement policies for Cutaneous T-Cell Lymphoma treatments have also been a significant driver for the growth of the CTCL market. In many developed countries, CTCL is recognized as a rare and serious medical condition, and governments and healthcare systems have implemented policies to ensure access to effective CTCL treatments. These policies often include coverage for diagnostic tests, approved therapies, and supportive care, reducing the financial burden on patients and encouraging the adoption of CTCL treatments. The availability of reimbursement has made CTCL treatments more accessible, particularly for patients with limited financial resources, and has contributed to the expansion of the CTCL market.
Market Opportunities:
Untapped Potential in Emerging Markets
The Cutaneous T-Cell Lymphoma market presents significant growth opportunities in emerging markets, such as Asia-Pacific and Latin America. These regions have a large and underserved patient population, with limited access to advanced diagnostic and treatment options for CTCL. As healthcare infrastructure and awareness of CTCL improve in these regions, the demand for effective CTCL therapies is expected to rise substantially. Pharmaceutical and biotechnology companies can capitalize on this untapped potential by expanding their geographic footprint, tailoring their product portfolios to meet regional needs, and collaborating with local healthcare providers to improve CTCL diagnosis and management. Investing in these emerging markets can open new avenues for growth and solidify the CTCL market's position as a global healthcare priority.
Expansion of Clinical Trials and Pipeline Products
The CTCL market is poised for further growth through the expansion of clinical trials and the development of new pipeline products. Pharmaceutical and biotechnology companies are actively investing in research and development to bring innovative CTCL therapies to the market. These efforts have resulted in a robust pipeline of targeted therapies, immunotherapies, and combination treatments that have the potential to improve patient outcomes and provide more effective disease management options. By expanding clinical trials to include diverse patient populations and explore new treatment approaches, companies can uncover valuable insights, optimize treatment strategies, and accelerate the approval and commercialization of these promising pipeline products, driving the growth of the CTCL market.
Leveraging Digital Technologies for Disease Management
The CTCL market can also benefit from the integration of digital technologies to enhance disease management and patient care. The use of telemedicine, mobile health applications, and remote patient monitoring can improve access to CTCL specialists, facilitate early diagnosis, and enable continuous monitoring of disease progression and treatment response. These digital solutions can also empower patients to actively engage in their care, improve adherence to therapies, and facilitate communication with healthcare providers. By leveraging these digital capabilities, CTCL market players can enhance the overall patient experience, improve treatment outcomes, and expand the reach of CTCL care, ultimately driving the growth of the market.
Collaboration and Partnerships for Innovation
Opportunities for growth in the CTCL market also lie in the fostering of collaborative efforts and strategic partnerships. By collaborating with academic institutions, research organizations, and other industry players, CTCL market participants can leverage diverse expertise, share resources, and accelerate the development of innovative treatments. These partnerships can facilitate the exchange of knowledge, the exploration of novel therapeutic approaches, and the optimization of clinical trial design and patient recruitment. Furthermore, collaborations can enable market players to expand their geographic reach, access new patient populations, and capitalize on complementary capabilities, ultimately driving the advancement of the CTCL market.
Market Trends:
Shift Towards Personalized Medicine and Precision Oncology
The Cutaneous T-Cell Lymphoma market is witnessing a significant shift towards personalized medicine and precision oncology. The increasing understanding of the genetic and molecular mechanisms underlying CTCL has enabled the development of targeted therapies that are tailored to the unique characteristics of individual patients. By leveraging genetic profiling, biomarker analysis, and advanced diagnostic tools, healthcare providers can now identify specific molecular targets and select the most appropriate treatment regimens for CTCL patients. This personalized approach aims to maximize the efficacy of CTCL therapies, minimize the risk of adverse events, and improve overall patient outcomes. As the focus on personalized medicine continues to grow, the CTCL market is likely to see an increased adoption of these precision-based treatment strategies, driving the market's expansion.
Rising Demand for Early Diagnosis and Effective Treatment Options
The Cutaneous T-Cell Lymphoma market is also witnessing a trend towards the increasing demand for early diagnosis and effective treatment options. Patients and healthcare providers alike are emphasizing the importance of timely detection and intervention, as early-stage CTCL is often more responsive to treatment and can lead to better long-term outcomes. The availability of advanced diagnostic techniques, as mentioned earlier, has contributed to this trend by enabling earlier identification of CTCL. Furthermore, the development of innovative therapies, such as targeted agents and immunotherapies, has provided patients with more effective treatment options, fueling the demand for these advanced solutions. As the market continues to evolve, the focus on early diagnosis and the provision of cutting-edge CTCL treatments will likely intensify, driving the growth of the CTCL market.
Emphasis on Developing Novel and Innovative Therapies
The Cutaneous T-Cell Lymphoma market is characterized by an increasing emphasis on the development of novel and innovative therapies. Pharmaceutical and biotechnology companies are actively investing in research and development to bring new and improved CTCL treatments to the market. This trend is driven by the need to address the limitations of existing therapies, such as suboptimal efficacy, adverse effects, and the development of drug resistance. The industry is exploring various therapeutic approaches, including targeted therapies, immunotherapies, combination treatments, and novel delivery mechanisms, to provide more effective and well-tolerated options for CTCL patients. As these innovative therapies enter the market and demonstrate their clinical benefits, the CTCL market is poised to experience significant growth and transformation.
Increasing Collaborations and Partnerships
The Cutaneous T-Cell Lymphoma market is witnessing a trend of increasing collaborations and partnerships among various stakeholders, including pharmaceutical companies, biotechnology firms, research organizations, and healthcare providers. These collaborative efforts aim to leverage complementary expertise, share resources, and accelerate the development and commercialization of CTCL therapies. By working together, market participants can explore synergistic opportunities, gain access to new patient populations, and expand their geographic reach. Furthermore, these partnerships can foster the exchange of knowledge, facilitate the translation of scientific discoveries into practical applications, and ultimately drive innovation in the CTCL market. As the industry continues to recognize the value of collaborative approaches, the CTCL market is likely to experience a surge in strategic alliances and partnerships, propelling its growth and transformation.
Market Restraints:
Rarity of the Disease and Limited Patient Population
One of the key restraints in the Cutaneous T-Cell Lymphoma market is the rarity of the disease and the limited patient population. CTCL is considered a rare disease, with a global incidence estimated to be around 3.9 per 100,000 individuals. The small patient pool poses a significant challenge for pharmaceutical and biotechnology companies, as it can be difficult to enroll a sufficient number of patients in clinical trials, which can slow down the development and approval of new CTCL therapies. Additionally, the limited market size and potential revenue opportunities may discourage some companies from investing in CTCL research and development, as they may prioritize larger and more commercially viable disease areas. This rarity and limited patient population remain a substantial restraint in the growth of the CTCL market.
High Cost of Advanced CTCL Treatments
The high cost of advanced Cutaneous T-Cell Lymphoma treatments is another significant restraint for the CTCL market. The development of innovative, targeted therapies and immunotherapies for CTCL often involves complex and costly research and manufacturing processes, which are ultimately reflected in the prices of these treatments. The financial burden associated with CTCL therapies can be a barrier for patients, particularly in regions with limited healthcare coverage or reimbursement. This high cost of treatment can limit access to CTCL care, leading to disparities in patient outcomes and potentially hindering the broader adoption of these advanced therapies. Addressing the issue of treatment affordability will be crucial for expanding the reach of the CTCL market and ensuring equitable access to CTCL care.
Side Effects Associated with CTCL Therapies
The side effects associated with Cutaneous T-Cell Lymphoma therapies also pose a restraint for the CTCL market. Many of the existing CTCL treatments, including chemotherapy, radiation, and even some targeted therapies, can cause significant adverse effects, such as skin irritation, fatigue, nausea, and an increased risk of infections. These side effects can negatively impact patients' quality of life, leading to treatment discontinuation, reduced adherence, and suboptimal clinical outcomes. While the development of more targeted and less toxic therapies has helped mitigate these issues, the persistent risk of side effects remains a concern for both patients and healthcare providers, potentially limiting the adoption of CTCL treatments and restricting the growth of the CTCL market.
Recent Developments:
|
Development |
Company Name |
|
In September 2021, the U.S. Food and Drug Administration (FDA) approved Poteligeo (mogamulizumab) for the treatment of adult patients with relapsed or refractory CTCL. This monoclonal antibody therapy has significantly impacted the CTCL market. |
Kyowa Kirin Co., Ltd. |
|
In March 2022, Miragen Therapeutics announced positive topline results from a Phase 2 clinical trial evaluating cobomarsen, an investigational microRNA therapeutics, for the treatment of CTCL. This promising candidate is expected to expand the treatment options for CTCL patients. |
Miragen Therapeutics, Inc. |
|
In June 2021, Actelion Pharmaceuticals (a Janssen Pharmaceutical Company) received FDA approval for Valchlor (mechlorethamine) gel, a topical therapy for the treatment of stage IA and IB CTCL. This approval has strengthened the company's presence in the CTCL market. |
Actelion Pharmaceuticals Ltd. (a Janssen Pharmaceutical Company) |
|
In November 2020, Sanofi announced the acquisition of Principia Biopharma, a biopharmaceutical company developing BTK inhibitors, including the investigational CTCL therapy SAR442168. This acquisition is expected to bolster Sanofi's oncology pipeline and expand its presence in the CTCL market. |
Sanofi |
|
In September 2019, Merck & Co., Inc. entered into a strategic collaboration with Ellipses Pharma to develop novel targeted therapies for various cancers, including CTCL. This partnership aims to leverage Merck's expertise in oncology and Ellipses Pharma's drug discovery platform. |
Merck & Co., Inc. |
Market Regional Insights:
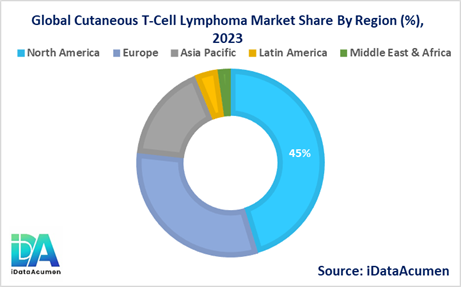
The Cutaneous T-Cell Lymphoma market is analyzed across five major regions: North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
North America is expected to be the largest market for Cutaneous T-Cell Lymphoma, accounting for over 45.2% of the global market share in 2024. The growth of the market in North America is attributed to the high incidence and prevalence of CTCL, well-established healthcare infrastructure, and the presence of key market players.
Europe is the second-largest market, contributing to 31.4% of the global market share in 2024. The growth in the European market is driven by factors such as the increasing awareness of CTCL, favorable reimbursement policies, and the availability of advanced diagnostic and treatment options.
The Asia Pacific region is expected to be the fastest-growing market for Cutaneous T-Cell Lymphoma, with a CAGR of over 14.9% during the forecast period. The growth in this region is attributed to the rising incidence of CTCL, improving healthcare infrastructure, and the increasing adoption of new treatments.
Market Segmentation:
- By Product Type
- Topical Therapies
- Systemic Therapies
- Phototherapy
- Combination Therapies
- Others (e.g., Immunotherapies, Targeted Therapies)
- By Disease Type
- Mycosis Fungoides
- Sézary Syndrome
- Primary Cutaneous CD30+ T-Cell Lymphoproliferative Disorders
- Cutaneous T-Cell Lymphoma, Unspecified
- Others (e.g., Primary Cutaneous Anaplastic Large Cell Lymphoma, Subcutaneous Panniculitis-Like T-Cell Lymphoma)
- By End-User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Home Care Settings
- Others (e.g., Research Institutes, Academic Institutions)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Mail Order Pharmacies
- Others (e.g., Specialty Pharmacies)
- By Route of Administration
- Topical
- Oral
- Intravenous
- Subcutaneous
- Others (e.g., Intramuscular, Intrathecal)
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East, and Africa
Segment Analysis:
The topical therapies segment is projected to be the fastest-growing segment in the Cutaneous T-Cell Lymphoma market, with a CAGR of 10.8% from 2024 to 2031. This growth is attributed to the increasing adoption of topical treatments, such as mechlorethamine gel (Valchlor) and other emerging topical agents, as the first-line approach for managing early-stage CTCL.
The systemic therapies segment is expected to be the largest segment, accounting for approximately 35% of the market share in 2024. The growth in this segment is driven by the availability of approved systemic treatments, such as Poteligeo (mogamulizumab), and the development of novel targeted therapies and immunotherapies that can provide more effective and durable responses for CTCL patients.
The Asia Pacific region is anticipated to be the fastest-growing market for the topical therapies segment, with a CAGR of 15.2% during the forecast period. This growth is attributed to the increasing awareness of CTCL, the improving healthcare infrastructure, and the rising adoption of innovative treatment options in the region.
Top Companies in the Cutaneous T-Cell Lymphoma Market:
- Kyowa Kirin Co., Ltd.
- Miragen Therapeutics, Inc.
- Actelion Pharmaceuticals Ltd. (a Janssen Pharmaceutical Company)
- Sanofi
- Merck & Co., Inc.
- Novartis AG
- Hoffmann-La Roche Ltd.
- AbbVie Inc.
- Pfizer Inc.
- Seattle Genetics, Inc.
- Celgene Corporation (a Bristol-Myers Squibb Company)
- Takeda Pharmaceutical Company Limited
- Gilead Sciences, Inc.
- Spectrum Pharmaceuticals, Inc.
- Innate Pharma SA
- EUSA Pharma (UK) Limited
- Medac GmbH
- Soligenix, Inc.
- Avadel Pharmaceuticals plc
- Amgen Inc.