Market Analysis:
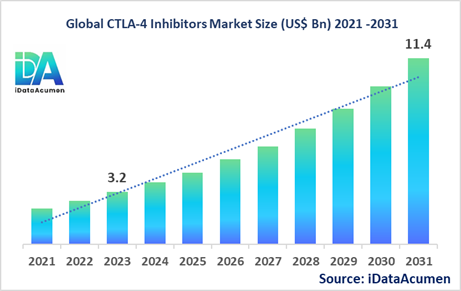
The CTLA-4 Inhibitors Market had an estimated market size of USD 3.2 billion in 2022, and it is projected to reach a global market valuation of USD 11.4 billion by 2030, growing at a CAGR of 17.2% from 2023 to 2030.
CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) inhibitors are a class of immunotherapy drugs that work by blocking the CTLA-4 receptor on T cells, thereby enhancing the immune system's ability to recognize and attack cancer cells. These drugs have revolutionized cancer treatment by harnessing the body's own immune defenses, offering a more targeted and potentially less toxic approach compared to traditional chemotherapy. The advantages of CTLA-4 inhibitors include improved survival rates, better quality of life, and the potential for long-lasting remissions in certain types of cancers.
The key drivers of the CTLA-4 Inhibitors Market include the rising prevalence of cancer globally, increasing adoption of immunotherapies, and a favorable regulatory environment that facilitates the development and approval of innovative cancer treatments.
In summary, CTLA-4 inhibitors represent a promising class of cancer immunotherapies that have demonstrated remarkable clinical efficacy and are poised for significant growth in the coming years.
The CTLA-4 Inhibitors Market is segmented by product type, indication, and region. By product type, the market is segmented into monoclonal antibodies and small molecules. The monoclonal antibodies segment is expected to dominate the market due to the approval of several well-established CTLA-4 inhibitors, such as ipilimumab (Yervoy), in this category. These drugs have shown remarkable efficacy in treating various types of cancers, including melanoma, lung cancer, and renal cell carcinoma.
A notable recent development in this segment is the approval of Libtayo (cemiplimab-rwlc) by the U.S. Food and Drug Administration (FDA) in February 2023 for the treatment of advanced non-small cell lung cancer (NSCLC) in certain patient populations.
Epidemiology Insights:
- The disease burden of cancers targeted by CTLA-4 inhibitors varies across major regions. North America and Europe have historically had higher incidence rates of melanoma, lung cancer, and other solid tumors, while the Asia-Pacific region is witnessing a rapid rise in cancer cases due to factors like aging populations and lifestyle changes.
- Key epidemiological trends and driving factors include the increasing prevalence of risk factors like smoking, obesity, and environmental exposures, as well as improved cancer screening and diagnosis methods. Additionally, demographic shifts, such as aging populations in developed nations, contribute to the rising incidence of cancer globally.
- According to the World Health Organization (WHO), in 2020, there were approximately 19.3 million new cancer cases and nearly 10 million cancer-related deaths worldwide. The most common cancers are lung, breast, colorectal, prostate, and stomach cancers.
- The increasing patient population, particularly in emerging markets, presents growth opportunities for CTLA-4 inhibitors and other cancer immunotherapies, as these treatments are often more effective and better tolerated compared to traditional cytotoxic chemotherapies.
- While cancers targeted by CTLA-4 inhibitors are not considered rare diseases, there are certain subpopulations or genetic mutations that may qualify for rare disease designations, potentially enabling faster regulatory approvals and market exclusivity for targeted therapies.
Market Landscape:
- Despite the success of CTLA-4 inhibitors, there are still unmet needs in the market. Many patients do not respond to these therapies, and there is a need for predictive biomarkers to identify potential responders. Additionally, the development of resistance mechanisms and the management of adverse events remain challenges.
- Current treatment options and approved CTLA-4 inhibitors include ipilimumab (Yervoy) from Bristol-Myers Squibb, which was the first CTLA-4 inhibitor approved for the treatment of metastatic melanoma. Tremelimumab, another CTLA-4 inhibitor from AstraZeneca, has shown promise in clinical trials for various cancer types.
- Upcoming therapies and technologies in the CTLA-4 inhibitor space include the development of next-generation CTLA-4 inhibitors with improved efficacy and safety profiles, as well as combination therapies with other immunotherapies, targeted therapies, or chemotherapies.
- Some breakthrough treatment options currently in development include bispecific antibodies that target both CTLA-4 and other immune checkpoints, such as PD-1 or PD-L1, as well as novel formulations and delivery methods for CTLA-4 inhibitors to improve their bioavailability and targeting.
- The CTLA-4 Inhibitors Market is dominated by branded drug manufacturers, with a few major pharmaceutical companies holding most of the market share. However, as patents expire, there is potential for generic drug manufacturers to enter the market, which could lead to increased competition and lower drug prices.
Market Report Scope:
|
Description |
|
|
The market size in 2023 |
US$ 3.2 Bn |
|
CAGR (2024 - 2031) |
17.2% |
|
The revenue forecast in 2031 |
US$ 11.4 Bn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Bristol-Myers Squibb, Merck & Co., Roche, AstraZeneca, Pfizer, Novartis, Sanofi, GlaxoSmithKline, Janssen Pharmaceuticals (Johnson & Johnson), Amgen, Eli Lilly and Company, Regeneron Pharmaceuticals, Celgene Corporation, Takeda Pharmaceutical Company, Gilead Sciences, Incyte Corporation, AbbVie, Daiichi Sankyo, Eisai Co., Ltd., Boehringer Ingelheim |
Market Drivers:
Increasing Prevalence of Cancer Globally
The rising incidence of various types of cancers worldwide is a significant driver for the growth of the CTLA-4 Inhibitors Market. According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, with nearly 10 million cancer-related deaths reported in 2020. The increasing prevalence can be attributed to factors such as aging populations, unhealthy lifestyles, and exposure to environmental carcinogens. As the cancer burden continues to grow, the demand for effective and innovative treatment options like CTLA-4 inhibitors is expected to rise substantially.
Favorable Regulatory Environment
The CTLA-4 Inhibitors Market is benefiting from a supportive regulatory environment, particularly in developed markets like the United States and Europe. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have implemented expedited review processes and special designations to facilitate the development and approval of innovative cancer therapies, including CTLA-4 inhibitors. This favorable regulatory landscape has encouraged pharmaceutical companies to invest in research and development, ultimately driving the growth of the market.
Increasing Adoption of Immunotherapies
The growing acceptance and adoption of immunotherapies, including CTLA-4 inhibitors, as a promising approach to cancer treatment is a significant driver for the market. Immunotherapies have demonstrated remarkable clinical efficacy in treating various types of cancers, offering potential for durable responses and improved survival rates. As healthcare professionals and patients become more aware of the benefits of immunotherapies, the demand for CTLA-4 inhibitors is expected to increase substantially.
Promising Clinical Trial Results
Ongoing clinical trials for CTLA-4 inhibitors have yielded promising results, fueling the growth of the market. These trials have demonstrated the efficacy of CTLA-4 inhibitors in treating various cancers, either as monotherapies or in combination with other immunotherapies or targeted therapies. Positive clinical trial outcomes have not only led to regulatory approvals but have also generated confidence among healthcare professionals and patients, driving the adoption of CTLA-4 inhibitors in clinical practice.
Market Opportunities:
Expansion into New Indications
The CTLA-4 Inhibitors Market presents an opportunity for expansion into new indications beyond the currently approved cancer types. As research and development efforts continue, CTLA-4 inhibitors may demonstrate efficacy in treating other types of cancers or even non-oncological indications. This potential for broader application could open up new market avenues and drive significant growth in the CTLA-4 Inhibitors Market.
Development of Combination Therapies
The potential for combining CTLA-4 inhibitors with other immunotherapies, targeted therapies, or chemotherapies presents a significant opportunity for the market. Combination therapies have shown promising results in clinical trials, offering the possibility of synergistic effects and improved efficacy compared to monotherapies. As more combination therapies are developed and approved, the CTLA-4 Inhibitors Market is expected to witness substantial growth.
Personalized Medicine and Biomarker-Driven Treatments
The increasing focus on personalized medicine and biomarker-driven treatments presents an opportunity for the CTLA-4 Inhibitors Market. By identifying specific biomarkers or genetic profiles associated with better response rates to CTLA-4 inhibitors, healthcare providers can optimize treatment strategies and improve patient outcomes. This targeted approach could lead to increased adoption of CTLA-4 inhibitors and drive market growth.
Emerging Markets and Healthcare Access
The expansion of healthcare access and increasing affordability of cancer treatments in emerging markets like Asia, Latin America, and Africa present a significant opportunity for the CTLA-4 Inhibitors Market. As these regions continue to develop their healthcare infrastructure and implement favorable reimbursement policies, the demand for advanced cancer therapies, including CTLA-4 inhibitors, is expected to rise, driving market growth.
Market Trends:
Emphasis on Combination Immunotherapies
A notable trend in the CTLA-4 Inhibitors Market is the increasing emphasis on combination immunotherapies. Researchers and pharmaceutical companies are exploring the potential of combining CTLA-4 inhibitors with other immune checkpoint inhibitors, such as PD-1 or PD-L1 inhibitors, to achieve synergistic effects and improve treatment outcomes. This trend has led to the development of several combination therapies currently in clinical trials or already approved for certain cancer types.
Focus on Predictive Biomarkers and Patient Selection
Another trend shaping the CTLA-4 Inhibitors Market is the focus on identifying predictive biomarkers and selecting patients most likely to respond to these therapies. By leveraging advanced diagnostic techniques and genomic profiling, healthcare providers can better identify patients who are more likely to benefit from CTLA-4 inhibitors, thereby improving treatment efficacy and reducing the risk of adverse events.
Development of Novel Formulations and Delivery Methods
The CTLA-4 Inhibitors Market is witnessing a trend towards the development of novel formulations and delivery methods for these therapies. Researchers are exploring strategies such as nanoparticle-based delivery systems, antibody-drug conjugates, and alternative routes of administration to improve the bioavailability, targeting, and therapeutic efficacy of CTLA-4 inhibitors.
Emphasis on Real-World Evidence and Patient-Reported Outcomes
There is an increasing emphasis on collecting and analyzing real-world evidence and patient-reported outcomes in the CTLA-4 Inhibitors Market. This trend is driven by the need to better understand the effectiveness, safety, and patient experience of these therapies in real-world settings. By incorporating this data, pharmaceutical companies and healthcare providers can make more informed decisions about treatment strategies and improve patient care.
Market Restraints
High Treatment Costs and Affordability Challenges
One of the significant restraints for the CTLA-4 Inhibitors Market is the high treatment costs associated with these therapies. CTLA-4 inhibitors are typically expensive, and the overall cost of treatment, including associated healthcare services, can be a significant financial burden for patients and healthcare systems, particularly in developing countries or regions with limited healthcare resources.
Adverse Effects and Toxicity Concerns
Despite their efficacy, CTLA-4 inhibitors are associated with potential adverse effects and toxicity concerns. These therapies can trigger immune-related adverse events, such as autoimmune disorders, rash, colitis, and endocrine abnormalities, which can be severe in some cases. The management of these adverse effects requires careful monitoring and additional interventions, which can add to the overall treatment burden and costs.
Resistance Mechanisms and Limited Patient Response Rates
While CTLA-4 inhibitors have demonstrated remarkable success in certain patient populations, a significant portion of patients may not respond to these therapies or develop resistance over time. The underlying mechanisms of resistance are not fully understood, and overcoming this challenge remains a significant restraint for the market's growth. Ongoing research efforts are focused on identifying biomarkers and developing strategies to overcome resistance and improve response rates.
Recent Developments:
|
Development |
Involved Company |
|
Libtayo (cemiplimab-rwlc) was approved by the FDA in February 2023 for the treatment of advanced non-small cell lung cancer in certain patient populations. This approval expands the use of Libtayo, a CTLA-4 inhibitor, in lung cancer treatment. |
Regeneron Pharmaceuticals, Inc. and Sanofi |
|
In September 2022, the FDA granted accelerated approval to Opdualag (nivolumab and relatlimab-rmbw), a fixed-dose combination of two immunotherapies, for the treatment of unresectable or metastatic melanoma. Relatlimab is a CTLA-4 inhibitor, while nivolumab targets the PD-1 checkpoint. |
Bristol-Myers Squibb |
|
In April 2022, the FDA approved Yervoy (ipilimumab) for the adjuvant treatment of adult patients with resected stage IIB/C melanoma, expanding the use of this CTLA-4 inhibitor in earlier stages of melanoma. |
Bristol-Myers Squibb |
|
Yervoy (ipilimumab) was approved by the FDA in March 2022 for the treatment of unresectable or metastatic melanoma in pediatric patients aged 12 years and older. This approval provides a new treatment option for this patient population. |
Bristol-Myers Squibb |
|
In February 2022, the FDA approved Yervoy (ipilimumab) in combination with Opdivo (nivolumab) for the treatment of unresectable advanced, recurrent or metastatic esophageal squamous cell carcinoma after prior fluoropyrimidine- and platinum-based chemotherapy. |
Bristol-Myers Squibb |
|
In January 2022, AstraZeneca announced positive results from the TROPION-Lung08 Phase 3 trial, evaluating the combination of tremelimumab (a CTLA-4 inhibitor) and Imfinzi (durvalumab, a PD-L1 inhibitor) in patients with metastatic non-small cell lung cancer. |
AstraZeneca |
|
In December 2021, Merck acquired Acceleron Pharma, a biopharmaceutical company, for $11.5 billion. This acquisition aimed to strengthen Merck's pipeline and portfolio in cardiovascular disease and other therapeutic areas. |
Merck & Co., Inc. and Acceleron Pharma Inc. |
|
In November 2021, Gilead Sciences acquired Immunomedics, a leader in antibody-drug conjugate technology, for $21 billion. This acquisition expanded Gilead's oncology portfolio and provided access to Trodelvy (sacituzumab govitecan), a first-in-class antibody-drug conjugate. |
Gilead Sciences, Inc. and Immunomedics, Inc. |
|
In October 2021, Bristol-Myers Squibb and Eisai Co., Ltd. announced a global strategic collaboration to jointly develop and commercialize Eisai's antibody-drug conjugate (ADC) portfolio, including Lenvima (lenvatinib) and Keytruda (pembrolizumab) in combination. |
Bristol-Myers Squibb and Eisai Co., Ltd. |
Market Regional Insights:
The CTLA-4 Inhibitors Market is witnessing significant growth across various regions, driven by the increasing prevalence of cancer, favorable regulatory environments, and the adoption of innovative immunotherapies.
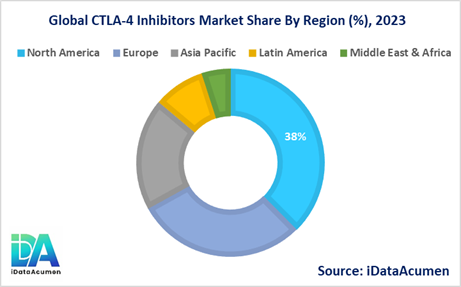
- North America is expected to be the largest market for the CTLA-4 Inhibitors Market during the forecast period, accounting for over 38% of the market share in 2024. The growth of the market in North America is attributed to the presence of key pharmaceutical companies, well-established healthcare infrastructure, and a higher cancer incidence rate.
- Europe is expected to be the second-largest market for the CTLA-4 Inhibitors Market, accounting for over 29.7% of the market share in 2024. The growth of the market in Europe is attributed to increasing research and development activities, favorable reimbursement policies, and a growing demand for personalized cancer treatments.
- The Asia-Pacific region is expected to be the fastest-growing market for the CTLA-4 Inhibitors Market, with a CAGR of over 19.4% during the forecast period by 2024. The growth of the market in the Asia-Pacific region is attributed to the rising cancer incidence, improving healthcare infrastructure, and increasing awareness about advanced cancer treatments. The market share of the Asia-Pacific region is estimated to be around 19.4% in 2024, making it the third-largest regional market.
Market Segmentation:
- By Product Type
- Monoclonal Antibodies
- Small Molecules
- Others (e.g., Antibody-Drug Conjugates, Bispecific Antibodies)
- By Indication
- Melanoma
- Non-Small Cell Lung Cancer
- Renal Cell Carcinoma
- Bladder Cancer
- Colorectal Cancer
- Others (e.g., Head and Neck Cancer, Ovarian Cancer, Breast Cancer)
- By Route of Administration
- Intravenous
- Subcutaneous
- Oral
- Others (e.g., Intramuscular, Topical)
- By Mechanism of Action
- Immune Checkpoint Inhibition
- T-Cell Activation
- Others (e.g., Antigen Presentation, Cytokine Modulation)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others (e.g., Specialty Pharmacies, Mail Order Pharmacies)
- By End User
- Hospitals
- Clinics
- Cancer Research Centers
- Others (e.g., Academic Institutions, Pharmaceutical Companies)
- By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Segment Analysis:
For the CTLA-4 Inhibitors Market, the monoclonal antibodies segment is projected to maintain its dominance and experience significant growth across all regions. This segment is expected to have the largest market size in 2024 due to the well-established efficacy and widespread adoption of monoclonal antibody CTLA-4 inhibitors like ipilimumab (Yervoy) and tremelimumab.
The monoclonal antibodies segment is projected to grow at a CAGR of around 18-20% in North America and Europe during the forecast period, driven by the high cancer incidence rates, favorable reimbursement policies, and the availability of advanced healthcare infrastructure.
In the Asia-Pacific region, the monoclonal antibodies segment is anticipated to witness the highest growth, with a CAGR of approximately 22-24%. This growth can be attributed to the rapidly increasing cancer burden, improving healthcare access, and the rising adoption of innovative cancer therapies in countries like China, India, and Japan.
The small molecules segment, though smaller in size, is also expected to witness notable growth, particularly in developed markets like North America and Europe, due to ongoing research and development efforts in this area.
Top companies in the CTLA-4 Inhibitors Market:
- Bristol-Myers Squibb
- Merck & Co.
- Roche
- AstraZeneca
- Pfizer
- Novartis
- Sanofi
- GlaxoSmithKline
- Janssen Pharmaceuticals (Johnson & Johnson)
- Amgen
- Eli Lilly and Company
- Regeneron Pharmaceuticals
- Celgene Corporation (now part of Bristol-Myers Squibb)
- Takeda Pharmaceutical Company
- Gilead Sciences
- Incyte Corporation
- AbbVie
- Daiichi Sankyo
- Eisai Co., Ltd.
- Boehringer Ingelheim