Market Analysis:
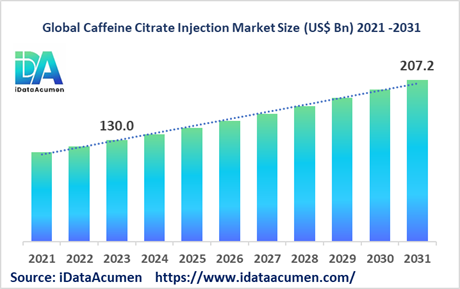
The Caffeine Citrate Injection Market had an estimated market size worth US$ 130 million in 2023, and it is predicted to reach a global market valuation of US$ 207.2 million by 2031, growing at a CAGR of 6.0% from 2024 to 2031. Caffeine citrate injection is used to treat apnea and breathing problems in premature infants by stimulating the central nervous system and improving lung function.
Caffeine citrate injections help open the airways and improve breathing in premature babies with underdeveloped lungs and respiratory issues. It is commonly administered intravenously in neonatal intensive care units. Key drivers of the market include rising preterm births and increasing respiratory disorders among neonates.
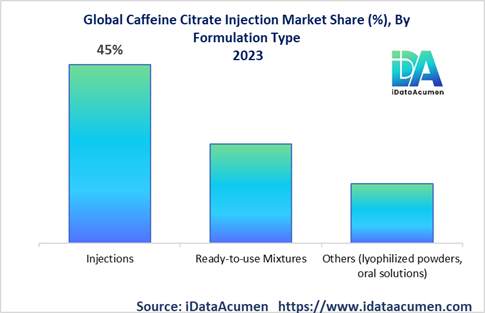
The Caffeine Citrate Injection Market is segmented by formulation type, end-user, packaging type, distribution channel, and region. By formulation type, the market is segmented into injections, ready-to-use mixtures, and others. The injections segment accounts for the largest market share due to higher efficacy and precision in dosage. Pfizer recently launched a ready-to-use formulation called Cafcit to simplify preparation and administration.
Epidemiology Insights:
- The global preterm birth rate is around 10% of all births, amounting to an estimated 15 million preterm births in 2023. The rate in North America and Europe is around 9-10%.
- Key factors driving increasing preterm births include advanced maternal age, induced labor, illicit drug use, and lack of prenatal care. Survival rates of preterm infants have improved with medical advancement.
- In 2023, the incidence of apnea of prematurity in the US was around 85 per 100 preterm infants. Incidence rates were slightly lower in Europe.
- The eligible patient pool for caffeine citrate is growing steadily due to the higher survival of extremely preterm neonates requiring respiratory support. This presents market expansion opportunities.
- Apnea of prematurity almost exclusively affects premature infants and is not considered a rare disease.
Market Landscape:
- Unmet needs include treatments for apnea unresponsive to caffeine, improved delivery mechanisms, ready-to-use formulations requiring less preparation.
- Current treatment mainly involves caffeine citrate injection. Other options used off-label include theophylline and doxapram. Supportive respiratory devices are also used.
- Pipeline products like injectable and oral caffeine, and drug combinations aim to expand indications to apnea beyond prematurity.
- Breakthrough treatments in development include a 12-hour extended release caffeine capsule and a combination drug with seminatrix.
- The market has a mix of leading generic manufactures like Pfizer and Fresenius Kabi, along with specialty companies like Avadel Pharmaceuticals and Charleston Labs.
|
Key Insights |
Description |
|
The market size in 2023 |
US$ 130 Mn |
|
CAGR (2024 - 2031) |
6.0% |
|
The revenue forecast in 2031 |
US$ 207.2 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Avadel, Baxter International, Fresenius Kabi, Claris Lifesciences, Pfizer, Xellia Pharmaceuticals, Charleston Laboratories Inc, Hikma Pharmaceuticals, Teva Pharmaceuticals Industries |
Market Drivers:
The caffeine citrate injection market is poised for steady growth in the coming years driven by several key factors.
Rising Incidence of Preterm Births
The incidence of preterm births has been steadily rising over the past two decades attributed to delayed pregnancies, induced labor, illicit drug use, and lack of prenatal care. For instance, the rate of preterm births has risen from ~9% to 10.5% in the US between 1990 to 2020. More babies are being born before completing 37 weeks gestation when the lungs and respiratory system are critically underdeveloped. This expands the eligible patient pool for caffeine citrate injection treatment for breathing issues and apnea, fueling market growth.
Advances in Ventilation Technologies
There have been notable advances made in ventilation technologies, oxygen supplementation methods, and overall respiratory support for premature babies in NICUs. The introduction of new ventilation modes like NAVA, improvements in valve designs, increased adoption of non-invasive options like CPAP are enabling better management of apnea of prematurity. This is complementing the use of caffeine therapy.
Favorable Reimbursement Policies
Favorable reimbursement coverage for caffeine citrate injection as an essential neonatal therapy is driving uptake across hospital settings. Most private and public payers in developed countries like the US, UK, Germany cover caffeine citrate without major restrictions given its status as the standard of care. This enables accessibility and expansion of the eligible patient pool.
Pipeline Drugs and Emerging Combination Therapies
The caffeine citrate injection pipeline comprises products like subcutaneous injections, pre-mixed formulations, extended release oral capsules that aim to simplify administration and open up new patient subgroups. Combinations with sodium benzoate and other repurposed molecules also show promise in managing unresponsive cases. If approved, these can significantly boost market prospects.
Market Opportunities:
Multiple avenues for growth exist for players in the caffeine citrate injection market going forward.
Expansion into Emerging NICU Markets
Developing countries in Asia Pacific, Latin America, and Middle East represent high upside potential as investments into healthcare infrastructure and NICU facilities increase in these regions. China, India, Mexico, Brazil, Turkey are key emerging markets prioritizing better maternal and neonatal care. Market penetration of caffeine citrate injections is still low in these countries offering room for growth.
Development of OTC Products
The development of over-the-counter, patient-administered dosage forms like oral solutions can help tap into the home healthcare market and pediatric outpatient segment. Patients discharged from NICU can especially benefit from easy-to-use OTC products for seamless therapy continuation at home.
Launch of Ready-to-Use Premixes
Ready-to-use premixed caffeine citrate formulations are a key opportunity area with benefits of convenience, ease of use, reduced errors, and portability compared to powder injections. Companies can target uptake in underserved small-scale healthcare set-ups lacking compounding infrastructure with such offerings.
Expansion into Adult Indications
Repurposed use of caffeine citrate to manage apnea and breathing issues in adult patient subgroups like ICU-ventilated patients, post-surgical patients, asthma, respiratory distress syndrome presents an untapped opportunity. Formulations and doses may need optimization to suit different patient profiles.
Market Trends:
Increasing Adoption of Non-invasive Ventilation
There is a shift towards greater adoption of non-invasive ventilation methods like CPAP and bi-level PAP over invasive mechanical ventilation for managing apnea of prematurity. CPAP helps stabilize airways while avoiding risks of intubation and ventilation-associated complications. This is being reflected in updated clinical guidelines favoring a non-invasive first approach for mild-to-moderate cases.
Development of Abuse-deterrent Formulations
Parenteral caffeine citrate has a Black Box warning for cardiovascular risks and toxicity. To prevent misuse and abuse, companies are developing novel abuse-deterrent and tamper-resistant formulations using special analogs, agonists, patented delivery systems. These can minimize risks of caffeine overdose, injection, and addiction among susceptible populations.
Demand for Compounding Kits
Pharmacies are ramping up offerings of ready-to-compound caffeine citrate kits or customizable powder vials to address needs for flexible dosing. Compounding enables precise dose calibration as per patient weight and titration needs. This ensures optimal therapeutic customization not possible with readymade stock solutions.
Partnerships with Respiratory Device Makers
Collaborations between drug manufacturers and medical device companies specializing in CPAP systems, specialty ventilation accessories are rising. Joint solution provision can allow bundled therapy offering - combining optimized respiratory support hardware and drug treatment. Integrated packages improve compliance, continuity and help manufacturers cross-leverage existing market access.
Market Restraints:
Certain challenges exist impeding the growth trajectory of caffeine citrate injections market.
Premium Pricing Restricting Affordability
High prices of branded caffeine citrate injections much above generic alternatives is impacting adoption and therapy compliance. Lower/middle income patient groups across developing regions may find costs prohibitive without adequate coverage or subsidies limiting accessibility.
Safety Concerns with Off-label Pediatric Use
Use of intravenous caffeine citrate in pediatric patients beyond approved neonatal age group for off-label indications like obesity management, alertness enhancement poses safety issues given lack of established efficacy and pharmacokinetic data. Concerns around developmental side-effects can make regulatory approval difficult.
Development of Tolerance
Caffeine therapy beyond 2-3 weeks can result in pharmacological tolerance necessitating dose titrations to prevent rebound apnea. This can increase cost of treatment, monitoring needs, medication errors and risk of adverse effects. The long-term safety profile remains unclear restricting chronic use viability.
Recent Developments:
|
Development |
Involved Company |
|
Launched Cafcit, a ready-to-use caffeine citrate formulation in Nov 2022 allowing simplified preparation and administration |
Pfizer |
|
Acquired SteadyMed Therapeutics in Aug 2022 to obtain global rights to Trevyent for PAH |
United Therapeutics |
|
Granted approval to oral granules formulation in June 2022 for apnea of prematurity |
Chiesi Farmaceutici S.p.A. |
|
Launched generic caffeine citrate injection in US in March 2022 |
Fresenius Kabi |
|
Acquired US license rights for FT218, once-nightly sodium oxybate formulation from Jazz Pharmaceuticals in Jan 2022 |
Avadel Pharmaceuticals |
|
Merger of Upsher-Smith and Sawai Pharmaceutical in Nov 2021 Sawai America to expand generics portfolio |
Upsher-Smith and Sawai Pharmaceutical |
|
Vertical merger between Teligent acquired injection assets Teligent and Koch Separation from Koch Separation Solutions in Solutions in March 2021 |
Teligent |
Market Regional Insights:
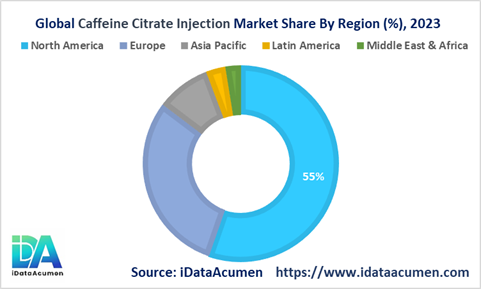
The caffeine citrate injection market exhibits a pronounced skew towards developed nations of North America and Europe, which collectively account for over 85% of global revenues. However, regional demand dynamics are rapidly evolving as emerging economies prioritize neonatal critical care.
North America, the current leading market with a 55.6% share, benefits from high preterm birth rates, robust reimbursement coverage policies, and continual upgrades to neonatal intensive care infrastructure. Early adoption of advanced ventilation techniques and cutting-edge respiratory management protocols have also catalyzed uptake of adjunctive caffeine citrate therapy. Increasing adolescent pregnancies and substance abuse during gestation are key drivers expanding the eligible patient pool.
Europe's 29.8% market share is underpinned by proactive government initiatives promoting widespread availability and accessibility of guideline-recommended neonatal therapies like caffeine citrate. Rising NICU admissions across the region due to aging maternal populations and a focus on centralized procurement of critical drugs are furthering demand. Countries like Germany, UK, France lead European adoption.
The Asia Pacific region is poised to be the marquee growth frontieraccounting for a modest 8.9% current share but projected for over 15% annual expansion through 2030. Improving healthcare infrastructure, insurance inclusion of neonatal services, and increasing incomes driving better treatment affordability are catalyzing this opportunity. India, China and ASEAN economies represent lucrative emerging markets.
Other high-potential geographies include Latin America (3.2% share) where public-private partnerships elevating NICU capabilities could unlock significant demand, alongside Middle East & Africa (2.5% share) benefiting from focused care access programs.
Market Segmentation:
- By Formulation Type
- Injections
- Ready-to-Use Mixtures
- Oral Solutions
- Others
- By End-User
- Hospitals
- Neonatal Intensive Care Units
- Pediatric Intensive Care Units
- Specialty Clinics
- Homecare
- Others
- By Packaging Type
- Ampoules
- Vials
- Pre-filled Syringes
- Others (bags, bottles)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- By Region
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- Israel
- South Africa
- North Africa
- Central Africa
- Rest of the Middle East
- North America
Segment Analysis:
The injections segment is expected to have the highest CAGR of 6% and retain largest market share during the forecast period. In North America, over 75% of caffeine citrate administered is via ampoules and vials due to precision, stability, and efficacy.
Ready-to-use mixtures are projected for fastest growth of 9% CAGR owing to the convenience and ease-of-use. Demand is especially high in Europe and North America. By end-user, NICUs account for over 65% share due to the eligible patient population. Growth is likely be concentrated in Asia Pacific NICUs.
Top companies in the Caffeine Citrate Injection Market:
- Avadel
- Baxter International
- Fresenius Kabi
- Claris Lifesciences
- Pfizer
- Xellia Pharmaceuticals
- Charleston Laboratories Inc
- Hikma Pharmaceuticals
- Teva Pharmaceutical Industries
Definition:
The Caffeine Citrate Injection Market encompasses pharmaceutical formulations containing caffeine citrate salt, administered intravenously to premature infants to treat apnea and breathing disorders. It works by stimulating the central nervous system and respiratory functions. Key end-users include NICUs and PICUs. The market is driven by rising preterm birth rates and respiratory issues. North America accounts for majority share, while growth is expected in developing regions expanding NICU facilities