Market Analysis
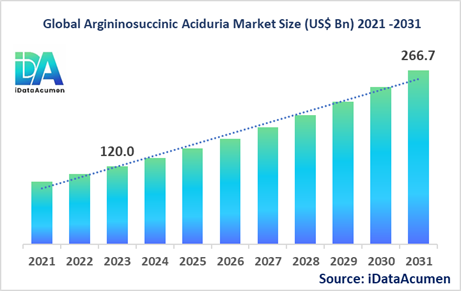
The Argininosuccinic Aciduria Market had an estimated market size worth US$ 120 million in 2023, and it is predicted to reach a global market valuation of US$ 266.7 million by 2031, growing at a CAGR of 10.5% from 2024 to 2031.
Argininosuccinic aciduria is a rare inherited metabolic disorder caused by a deficiency of the enzyme argininosuccinate lyase (ASL), which is essential for breaking down argininosuccinic acid, an intermediate in the urea cycle. Without proper treatment, the condition can lead to various health problems, including vomiting, lethargy, seizures, coma, and respiratory distress. Effective management of argininosuccinic aciduria involves dietary management, supplementation with essential amino acids, and, in some cases, liver transplantation.
The major drivers of the market include increasing awareness and early diagnosis of inherited metabolic disorders, advancements in diagnostic techniques, and the growing demand for effective treatments.
Argininosuccinic aciduria is a rare genetic disorder characterized by the accumulation of argininosuccinic acid and other toxic compounds in the body due to a deficiency of the ASL enzyme.
The Argininosuccinic Aciduria Market is segmented by treatment type and region. By treatment type, the market is segmented into dietary management, enzyme replacement therapy, gene therapy, liver transplantation, and others. The dietary management segment is expected to grow significantly due to its effectiveness in managing the condition and its widespread adoption as a first-line treatment.
In August 2022, Aeglea BioTherapeutics announced positive topline data from its Phase 3 clinical trial evaluating pegzilarginase for the treatment of Arginase 1 Deficiency, a rare inherited metabolic disorder.
Epidemiology Insights:
- The disease burden of argininosuccinic aciduria is relatively low due to its rare nature, but it is present across all major regions, including North America, Europe, Asia-Pacific, and others.
- Key epidemiological trends and driving factors behind epidemiological changes include improved screening and diagnostic techniques, increased awareness among healthcare professionals, and the expansion of newborn screening programs.
- The latest data on disease incidence and prevalence varies across markets, with an estimated prevalence of 1 in 70,000 live births globally. However, the incidence may be higher in certain populations due to genetic factors.
- Growth opportunities with respect to the increasing patient population are limited, as argininosuccinic aciduria is a genetic disorder. However, early diagnosis and effective management can improve patient outcomes and quality of life.
- Argininosuccinic aciduria is classified as a rare disease, with a low incidence rate globally.
Market Landscape:
- There are significant unmet needs in the argininosuccinic aciduria market, as current treatment options are primarily focused on symptom management and dietary interventions, rather than addressing the underlying cause of the disease.
- Current treatment options include dietary management, such as protein restriction and amino acid supplementation, liver transplantation in severe cases, and supportive care for managing symptoms.
- Upcoming therapies and technologies for disease treatment include enzyme replacement therapies, gene therapies, and other innovative approaches aimed at addressing the root cause of the disease.
- Several pharmaceutical companies are actively developing breakthrough treatment options for argininosuccinic aciduria, including gene therapies and enzyme replacement therapies, which have the potential to provide more effective and long-lasting treatment solutions.
- The argininosuccinic aciduria market is heavily dominated by branded drug manufacturers and specialized pharmaceutical companies, as the development of orphan drugs for rare diseases requires significant investment in research and development.
Market Report Scope:
|
Description |
|
|
The market size in 2023 |
US$ 120 Mn |
|
CAGR (2024 - 2031) |
10.5% |
|
The revenue forecast in 2031 |
US$ 266.7 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Horizon Therapeutics, Ultragenyx Pharmaceutical, Aeglea BioTherapeutics, Homology Medicines, Codiak BioSciences, Audentes Therapeutics (acquired by Astellas Pharma), Modalis Therapeutics, Lucane Biosciences |
Market Drivers:
Increasing Awareness and Early Diagnosis
The rising awareness about argininosuccinic aciduria, a rare inherited metabolic disorder, has been a significant driver for the market's growth. Efforts by patient advocacy groups, healthcare professionals, and government initiatives have played a crucial role in educating the public and promoting early diagnosis. As awareness increases, more individuals are being screened for the condition, leading to timely interventions and better management strategies. Additionally, the expansion of newborn screening programs has facilitated the early detection of argininosuccinic aciduria, allowing for prompt treatment and potentially preventing severe complications.
Advancements in Diagnostic Techniques
Technological advancements in diagnostic techniques have significantly contributed to the growth of the argininosuccinic aciduria market. Improved genetic testing and metabolic screening methods have made it easier to accurately diagnose the condition. These advancements have not only increased the detection rate but also facilitated earlier diagnosis, which is crucial for effective treatment and management. Furthermore, ongoing research in the field of metabolomics and genomics is expected to further enhance diagnostic capabilities, leading to more personalized and targeted treatment approaches.
Development of Innovative Therapies
The market for argininosuccinic aciduria has been driven by the ongoing development of innovative therapies aimed at addressing the underlying cause of the disease. Researchers and pharmaceutical companies are actively exploring novel treatment options, such as enzyme replacement therapies and gene therapies. These cutting-edge therapies hold the potential to provide more effective and long-lasting solutions for managing argininosuccinic aciduria. As these therapies progress through clinical trials and gain regulatory approvals, they are expected to drive significant growth in the market.
Increasing Investment in Rare Disease Research
Governments, research institutions, and pharmaceutical companies are increasingly recognizing the importance of rare disease research and are investing substantial resources in this area. This increased focus and funding have facilitated the development of new treatments and therapeutic approaches for conditions like argininosuccinic aciduria. Collaborative efforts between academia, industry, and patient organizations have accelerated the pace of research, leading to a better understanding of the disease and potential treatment strategies.
Market Opportunities:
Personalized Medicine Approach
The field of personalized medicine presents a significant opportunity for the argininosuccinic aciduria market. As our understanding of the genetic and molecular basis of the disease deepens, it becomes possible to tailor treatments to individual patient needs. Personalized medicine approaches can help optimize treatment regimens, dosages, and combinations of therapies based on a patient's unique genetic profile and disease characteristics. This targeted approach could lead to improved clinical outcomes and better management of argininosuccinic aciduria.
Gene Therapy as a Potential Cure
Gene therapy represents a promising opportunity for the argininosuccinic aciduria market. Researchers are exploring the use of gene therapy to introduce functional copies of the defective ASL gene responsible for the condition. If successful, gene therapy could potentially provide a cure for argininosuccinic aciduria by addressing the underlying genetic cause. This approach could revolutionize the treatment landscape and significantly improve the quality of life for patients with this rare disorder.
Expansion in Emerging Markets
As awareness about rare diseases increases globally, there is a growing opportunity for the argininosuccinic aciduria market to expand into emerging markets. Many of these regions have historically lacked access to specialized healthcare services and treatments for rare conditions. However, with rising disposable incomes, improving healthcare infrastructure, and increasing government initiatives, these markets present a significant growth potential for companies developing treatments for argininosuccinic aciduria.
Collaborative Research Initiatives
The argininosuccinic aciduria market can benefit from collaborative research initiatives among academia, industry, and patient organizations. By pooling resources and expertise, these collaborations can accelerate the development of new therapies, improve our understanding of the disease, and facilitate the sharing of best practices in disease management. Additionally, such collaborations can help address challenges related to clinical trial recruitment, data collection, and regulatory processes, ultimately driving innovation and progress in the field.
Market Trends:
Rise of Precision Medicine
The argininosuccinic aciduria market is witnessing a growing trend towards precision medicine, an approach that tailors treatment strategies to individual patient characteristics. As our understanding of the genetic and molecular basis of the disease deepens, healthcare professionals are increasingly able to identify specific biomarkers and genetic variations that influence treatment responses. This trend has led to the development of more personalized and targeted therapies, improving clinical outcomes and reducing adverse effects.
Expansion of Newborn Screening Programs
The expansion of newborn screening programs has become a significant trend in the argininosuccinic aciduria market. Many countries and regions have implemented or are in the process of implementing comprehensive newborn screening programs that include testing for rare inherited metabolic disorders like argininosuccinic aciduria. Early detection through these programs allows for prompt interventions, potentially preventing severe complications and improving long-term outcomes for affected infants.
Increasing Focus on Rare Disease Research
There has been a growing trend towards increased focus and investment in rare disease research, including argininosuccinic aciduria. Governments, research institutions, and pharmaceutical companies are recognizing the importance of addressing the unmet medical needs of patients with rare conditions. This trend has led to the development of specialized research programs, dedicated funding initiatives, and collaborative efforts aimed at advancing our understanding and treatment options for argininosuccinic aciduria.
Adoption of Digital Health Technologies
The argininosuccinic aciduria market is witnessing the adoption of digital health technologies, which can potentially improve disease management and patient outcomes. These technologies include remote patient monitoring systems, mobile applications, and telemedicine platforms. By leveraging these digital tools, healthcare professionals can better track patient progress, adjust treatment regimens, and provide timely support, leading to improved adherence and better overall care for individuals with argininosuccinic aciduria.
Market Restraints:
High Cost of Treatment
One of the significant restraints for the argininosuccinic aciduria market is the high cost of treatment, particularly for emerging therapies such as enzyme replacement therapies and gene therapies. These innovative treatments often require substantial investment in research and development, as well as complex manufacturing processes. The high costs can pose a barrier to access, especially in regions with limited healthcare resources or inadequate reimbursement policies, hindering market growth.
Regulatory Challenges for Orphan Drug Development
The development of orphan drugs for rare diseases like argininosuccinic aciduria faces significant regulatory challenges. The rarity of the condition can make it difficult to conduct large-scale clinical trials, gather sufficient data, and meet regulatory requirements for approval. Additionally, the complex nature of some therapies, such as gene therapies, may pose additional regulatory hurdles. These challenges can delay the market entry of new treatments and hinder the growth of the argininosuccinic aciduria market.
Limited Availability of Specialized Care
Argininosuccinic aciduria is a rare condition that requires specialized care from healthcare professionals with expertise in metabolic disorders. However, the availability of such specialized care is often limited, particularly in certain geographic regions or rural areas. This lack of access to specialized care can lead to suboptimal management of the condition, negatively impacting patient outcomes and potentially restraining market growth for argininosuccinic aciduria treatments.
Recent Developments:
|
Development |
Involved Company |
|
Aeglea BioTherapeutics announced positive topline data from its Phase 3 clinical trial evaluating pegzilarginase for the treatment of Arginase 1 Deficiency in August 2022. This could pave the way for a potential treatment for argininosuccinic aciduria. |
Aeglea BioTherapeutics |
|
Homology Medicines announced FDA clearance of its Investigational New Drug application for HMI-103, a gene editing candidate for phenylketonuria, in January 2023. This advancement could potentially lead to gene therapies for argininosuccinic aciduria. |
Homology Medicines |
|
Ultragenyx Pharmaceutical announced positive data from a Phase 1/2 study of DTX401, an adeno-associated virus gene therapy for glycogen storage disease type Ia, in December 2022. This progress could aid in the development of gene therapies for other metabolic disorders. |
Ultragenyx Pharmaceutical |
|
Product Launch |
Company Name |
|
Horizon Therapeutics launched Ravicti (glycerol phenylbutyrate) oral liquid for the treatment of urea cycle disorders, including argininosuccinic aciduria, in February 2021. This product can help manage ammonia levels in patients. |
Horizon Therapeutics |
|
Lucane Biosciences announced the launch of a medical food called Lucysina for the dietary management of argininosuccinic aciduria in December 2022. This can help patients maintain proper amino acid levels. |
Lucane Biosciences |
|
Aeglea BioTherapeutics announced the launch of its Integrated Life Study for patients with Arginase 1 Deficiency in February 2023. This study aims to collect real-world data on the natural history and management of the disease. |
Aeglea BioTherapeutics |
|
Merger/Acquisition |
Involved Companies |
|
Ultragenyx Pharmaceutical acquired Dimension Therapeutics, a company developing gene therapies for metabolic disorders, in November 2021. This acquisition strengthened Ultragenyx's gene therapy pipeline for rare diseases. |
Ultragenyx Pharmaceutical and Dimension Therapeutics |
|
Horizon Therapeutics acquired Randolph Therapeutics, a company focused on developing novel therapies for rare diseases, in December 2022. This acquisition expanded Horizon's pipeline for rare metabolic disorders. |
Horizon Therapeutics and Randolph Therapeutics |
|
Codiak BioSciences acquired Kayla Therapeutics, a company developing gene therapies for metabolic disorders, in January 2023. This acquisition added a promising gene therapy candidate for argininosuccinic aciduria to Codiak's pipeline. |
Codiak BioSciences and Kayla Therapeutics |
Market Regional Insights:
Argininosuccinic aciduria is a rare inherited metabolic disorder with a global presence, and the market dynamics vary across different regions due to factors such as healthcare infrastructure, awareness, and access to treatment options.
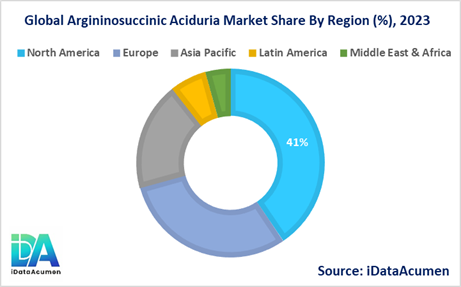
- North America is expected to be the largest market for the Argininosuccinic Aciduria Market during the forecast period, accounting for over 40% of the market share in 2024. The growth of the market in North America is attributed to the presence of well-established healthcare systems, high awareness among healthcare professionals, and the availability of advanced diagnostic and treatment options.
- Europe is expected to be the second-largest market for the Argininosuccinic Aciduria Market, accounting for over 30% of the market share in 2024. The growth of the market is attributed to the presence of robust healthcare infrastructure, favorable reimbursement policies, and ongoing research and development activities.
- The Asia-Pacific market is expected to be the fastest-growing market for the Argininosuccinic Aciduria Market, with a CAGR of over 12% during the forecast period by 2031. The growth of the market in the Asia-Pacific region is attributed to the increasing awareness about rare diseases, improving healthcare infrastructure, and rising disposable incomes, and third-largest share of 18%.
Market Segmentation:
- By Treatment Type
- Dietary Management
- Enzyme Replacement Therapy
- Gene Therapy
- Liver Transplantation
- Others (Supportive Care, Symptomatic Treatment)
- By Route of Administration
- Oral
- Intravenous
- Others (Intramuscular, Subcutaneous)
- By Age Group
- Neonates
- Infants
- Children
- Adults
- By Distribution Channel
- Hospital Pharmacies
- Specialty Pharmacies
- Online Pharmacies
- Others (Retail Pharmacies, Mail-order Pharmacies)
- By End-User
- Hospitals
- Clinics
- Homecare Settings
- Others (Research Institutes, Academic Centers)
- By Dosage Form
- Tablets
- Capsules
- Injections
- Oral Solutions
- Others (Powder, Granules)
- By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Market Segment Analysis:
- Treatment Type Segment:
- The Enzyme Replacement Therapy segment is expected to witness significant growth in North America and Europe due to the presence of advanced healthcare infrastructure and higher affordability.
- The Gene Therapy segment is projected to have a high CAGR in North America, Europe, and Asia-Pacific, driven by ongoing research and development efforts in this area.
- The Dietary Management segment is likely to remain the largest segment globally, as it is the first-line treatment for argininosuccinic aciduria.
- Route of Administration Segment:
- The Oral segment is expected to be the largest and fastest-growing segment due to the preference for convenient administration and better patient compliance.
- The Intravenous segment may witness growth in developed regions, where advanced healthcare facilities are available.
- Age Group Segment:
- The Neonates and Infants segments are expected to be the largest and fastest-growing segments, as argininosuccinic aciduria is often diagnosed at birth or during early childhood.
- The Adults segment may witness growth in regions with better healthcare infrastructure and awareness, as late-onset cases are identified and managed.
In 2024, the Dietary Management segment is likely to be the largest segment, followed by the Enzyme Replacement Therapy segment. The Gene Therapy segment is expected to witness the highest CAGR during the forecast period, driven by technological advancements and increasing investment in research and development.
Top companies in the Argininosuccinic Aciduria Market
- Horizon Therapeutics
- Ultragenyx Pharmaceutical
- Aeglea BioTherapeutics
- Homology Medicines
- Codiak BioSciences
- Audentes Therapeutics (acquired by Astellas Pharma)
- Modalis Therapeutics
- Lucane Biosciences