Market Analysis:
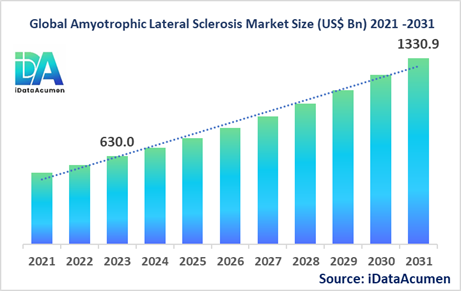
The Amyotrophic Lateral Sclerosis (ALS) Market had an estimated market size worth US$ 630 million in 2023, and it is predicted to reach a global market valuation of US$ 1,330.9 million by 2031, growing at a CAGR of 9.8% from 2024 to 2031.
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive neurodegenerative disorder that affects the motor neurons responsible for controlling voluntary muscle movement. The disease leads to muscle weakness, atrophy, and eventual paralysis, making it a debilitating condition for patients. Treatment options for ALS are primarily focused on symptom management and improving quality of life, as there is currently no cure available.
The key drivers of the ALS market include the increasing prevalence of the disease due to an aging population, improved diagnostic techniques, and growing research and development efforts to develop effective treatments. The rising geriatric population globally is a significant contributor to the increasing disease burden, as the risk of developing ALS increases with age.
The Amyotrophic Lateral Sclerosis (ALS) Market is segmented by drug class, route of administration, distribution channel, end-user, and region. By drug class, the market is segmented into riluzole, edaravone, and others (neuroprotective agents, anti-inflammatory drugs). The riluzole segment is expected to hold a significant market share due to its widespread use as the only FDA-approved drug for ALS, slowing the progression of the disease.
The market has witnessed several notable product launches and advancements in recent years, such as the approval of Radicava (edaravone) by the FDA in 2017 for the treatment of ALS.
Epidemiology Insights:
- The disease burden of ALS varies across major regions, with higher incidence rates observed in North America and Europe compared to other regions. According to estimates, the annual incidence of ALS in the United States is approximately 5.2 per 100,000 population.
- Key epidemiological trends and driving factors behind epidemiological changes in major markets like the US, EU5 (France, Germany, Italy, Spain, and the United Kingdom), and Japan include the aging population, improved diagnostic techniques, and increased awareness about the disease.
- The latest epidemiological data suggests that the prevalence of ALS in the United States is approximately 5 cases per 100,000 population, with an estimated 20,000 individuals living with the disease at any given time.
- The increasing patient population due to the aging demographic presents growth opportunities for the ALS market, as more individuals become susceptible to the disease. However, the lack of effective treatments and the progressive nature of the condition pose challenges for the market.
- ALS is not considered a rare disease, as it affects a significant number of individuals globally. However, its relatively low prevalence compared to other neurodegenerative disorders highlights the need for continued research and development efforts.
Market Landscape:
- There are significant unmet needs in the ALS market, as the current treatment options are limited and primarily focused on symptom management rather than addressing the underlying causes of the disease.
- The only FDA-approved therapies for ALS are riluzole and edaravone (Radicava), which can modestly slow the progression of the disease but do not provide a cure.
- Several promising therapies and technologies are currently in development, including gene therapies, stem cell therapies, and neuroprotective agents aimed at targeting specific genetic mutations or cellular pathways involved in ALS.
- Breakthrough treatment options under development include antisense oligonucleotide therapies, such as tofersen developed by Biogen and Ionis Pharmaceuticals, which targets specific genetic mutations associated with ALS.
- The ALS market is dominated by branded drug manufacturers, as the development of novel therapies for this complex disease requires significant investment in research and development efforts.
Market Report Scope:
|
Key Insights |
Description |
|
The market size in 2023 |
US$ 630 Mn |
|
CAGR (2024 - 2031) |
9.8% |
|
The revenue forecast in 2031 |
US$ 1,330.9 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Mitsubishi Tanabe Pharma Corporation, Biogen Inc., Ionis Pharmaceuticals, Inc., Cytokinetics, Inc., Orion Pharma, Apotex Inc., Sun Pharmaceutical Industries Ltd., Bausch Health Companies Inc. |
Market Drivers:
Increasing Prevalence of ALS
The rising prevalence of Amyotrophic Lateral Sclerosis (ALS) is a significant driver for the market's growth. ALS is a progressive neurodegenerative disorder that affects the motor neurons responsible for controlling voluntary muscle movement. As the global population ages, the risk of developing ALS increases, leading to a higher disease burden. Additionally, improved diagnostic techniques and increased awareness among healthcare professionals have contributed to better identification and diagnosis of ALS cases.
Recent studies have revealed a concerning trend of increasing ALS prevalence worldwide. For instance, a study published in the Journal of Neurology, Neurosurgery, and Psychiatry reported an annual increase of 2.7% in the prevalence of ALS in the United States between 2010 and 2015. This growing patient population presents a substantial market opportunity for pharmaceutical companies and healthcare providers focusing on ALS treatment and management.
Advancements in Diagnostic Techniques
Advancements in diagnostic techniques have played a crucial role in driving the ALS market by enabling earlier and more accurate diagnosis of the condition. Early diagnosis is essential for initiating prompt treatment and managing the disease progression more effectively. Innovative diagnostic methods, such as electromyography (EMG), neuroimaging techniques like magnetic resonance imaging (MRI), and the identification of specific biomarkers, have revolutionized the diagnostic process for ALS.
For instance, the development of neurofilament light chain (NfL) as a potential biomarker for ALS has shown promising results in identifying the disease at an early stage. These advancements not only facilitate timely diagnosis but also open up opportunities for clinical trials and the development of targeted therapies, fueling the growth of the ALS market.
Increasing Investment in Research and Development
The lack of effective treatments for ALS has driven significant investment in research and development efforts by pharmaceutical companies, academic institutions, and research organizations. The urgent need for disease-modifying therapies and potential cures has catalyzed extensive research initiatives worldwide.
Numerous clinical trials are currently underway, exploring various therapeutic approaches, including gene therapies, stem cell therapies, and neuroprotective agents. For example, companies like Biogen and Ionis Pharmaceuticals are conducting advanced clinical trials for their investigational drug tofersen, an antisense oligonucleotide targeting SOD1 mutations in ALS.
This continuous influx of investment and research efforts has the potential to yield breakthrough discoveries and novel treatments, driving the growth of the ALS market and providing hope for improved patient outcomes.
Growing Awareness and Support Initiatives
Increasing awareness about ALS and its debilitating effects has led to the establishment of various support initiatives and advocacy groups. These organizations play a vital role in driving the ALS market by promoting awareness, funding research, and providing resources for patients and caregivers.
Organizations such as the ALS Association, Muscular Dystrophy Association (MDA), and the International Alliance of ALS/MND Associations have been instrumental in raising awareness, supporting clinical trials, and advocating for better access to treatments and care services.
These initiatives have not only increased public understanding of ALS but have also attracted more research funding and encouraged collaboration among stakeholders, ultimately driving the growth of the ALS market.
Market Opportunities:
Development of Disease-Modifying Therapies
The development of disease-modifying therapies represents a significant opportunity for the ALS market. Currently, approved treatments for ALS primarily focus on symptom management and slowing disease progression, but there is a pressing need for therapies that can target the underlying causes of the disease and potentially halt or reverse its course.
Several pharmaceutical companies and research institutions are actively exploring various therapeutic approaches, such as gene therapies, stem cell therapies, and neuroprotective agents. For instance, Biogen and Ionis Pharmaceuticals are conducting clinical trials for tofersen, an investigational antisense oligonucleotide therapy targeting SOD1 mutations associated with ALS.
The successful development of disease-modifying therapies could revolutionize the ALS market by providing more effective treatment options and potentially extending the lifespan and quality of life for patients.
Personalized Medicine Approaches
With the increasing understanding of the genetic and molecular mechanisms underlying ALS, there is an opportunity to develop personalized medicine approaches tailored to individual patient characteristics. ALS is a heterogeneous disease with various genetic and environmental factors contributing to its development.
By leveraging advances in genomics, biomarker discovery, and precision medicine, researchers can identify specific genetic mutations or molecular pathways involved in different forms of ALS. This knowledge can pave the way for targeted therapies that address the specific underlying mechanisms for each patient.
Personalized medicine approaches have the potential to improve treatment outcomes by providing more effective and tailored interventions, thereby driving the growth of the ALS market.
Expansion into Emerging Markets
As awareness of ALS increases globally, there is an opportunity for the ALS market to expand into emerging markets. Many developing countries have limited access to advanced diagnostic techniques and treatment options for ALS, presenting an untapped market potential.
Pharmaceutical companies and healthcare providers can leverage partnerships and collaborations to establish a presence in these emerging markets, fostering improved access to ALS care and treatments. Additionally, emerging markets often have larger populations and a growing middle class, representing a significant patient base.
By addressing the unmet medical needs in these regions and providing affordable treatment options, companies can expand their market reach and contribute to improved patient outcomes worldwide.
Collaborative Research Initiatives
Collaborative research initiatives among academia, pharmaceutical companies, and nonprofit organizations present a promising opportunity for accelerating the development of new treatments and advancing the understanding of ALS.
By pooling resources, expertise, and data, collaborative efforts can expedite the translation of scientific discoveries into clinical applications. These collaborations can facilitate the sharing of knowledge, promote interdisciplinary approaches, and foster the development of innovative therapeutic strategies.
Furthermore, collaborative initiatives can help address the challenges associated with conducting clinical trials for ALS, a condition with a relatively small patient population, by leveraging combined resources and patient recruitment efforts.
Market Trends:
Emphasis on Early Diagnosis and Biomarker Development
The Amyotrophic Lateral Sclerosis (ALS) market is witnessing a growing emphasis on early diagnosis and biomarker development. Early detection of the disease is crucial for initiating prompt treatment and potentially slowing its progression. However, diagnosing ALS in its early stages can be challenging due to the subtle and nonspecific nature of the initial symptoms. Researchers are actively exploring various biomarkers, such as neurofilament light chain (NfL) levels, that could aid in the early and accurate diagnosis of ALS. These biomarkers can potentially help differentiate ALS from other neurodegenerative disorders and monitor disease progression.
The development and validation of reliable biomarkers not only facilitate early diagnosis but also support the evaluation of potential therapies in clinical trials, driving the growth of the ALS market. For instance, a recent study published in Nature Medicine demonstrated the potential of NfL as a promising biomarker for monitoring disease progression and assessing the efficacy of investigational therapies in ALS clinical trials.
Adoption of Precision Medicine Approaches
The Amyotrophic Lateral Sclerosis (ALS) market is witnessing a trend towards the adoption of precision medicine approaches, which involve tailoring treatments to individual patient characteristics. ALS is a heterogeneous disease, with various genetic and molecular factors contributing to its development and progression. By leveraging advances in genomics, proteomics, and bioinformatics, researchers can identify specific genetic mutations, molecular pathways, or disease subtypes associated with different forms of ALS. This knowledge can guide the development of targeted therapies that address the underlying mechanisms specific to each patient.
Precision medicine approaches have the potential to improve treatment outcomes by providing more effective and personalized interventions, thereby driving the growth and evolution of the ALS market. For example, researchers at the University of Michigan are exploring the use of precision medicine strategies to develop targeted therapies for ALS patients with specific genetic mutations.
Focus on Combination Therapies
Recognizing the complex and multifaceted nature of ALS, there is a growing trend towards exploring combination therapies that target multiple pathways and mechanisms involved in the disease. Rather than relying on a single therapeutic approach, researchers are investigating the potential synergistic effects of combining different treatment modalities. For instance, some clinical trials are evaluating the combination of neuroprotective agents with anti-inflammatory drugs or antioxidants. These combination therapies aim to address various aspects of the disease, such as neuronal degeneration, inflammation, oxidative stress, and protein misfolding.
By targeting multiple pathways simultaneously, combination therapies may provide enhanced therapeutic benefits and potentially lead to improved patient outcomes, driving the growth and innovation within the ALS market. A recent study published in the Journal of Neurology, Neurosurgery & Psychiatry explored the potential of combining riluzole and edaravone for the treatment of ALS, demonstrating promising results in slowing disease progression.
Increased Focus on Patient-Centric Care
The Amyotrophic Lateral Sclerosis (ALS) market is witnessing an increased focus on patient-centric care, which emphasizes the importance of addressing the comprehensive needs of patients and their caregivers. ALS is a debilitating condition that not only affects physical function but also has profound psychological and emotional impacts on individuals and their families. Patient-centric care approaches aim to provide holistic support, encompassing physical, emotional, and social aspects of care.
This trend has led to the development of multidisciplinary care teams, comprising healthcare professionals from various disciplines, such as neurologists, physical therapists, respiratory therapists, nutritionists, and social workers. These teams collaborate to develop personalized treatment plans and provide comprehensive support to patients and their caregivers throughout the course of the disease. The increased focus on patient-centric care has driven the growth of specialized ALS clinics and support services, further enhancing the overall market landscape.
Market Restraints:
High Cost of Treatment
The high cost of treatment associated with Amyotrophic Lateral Sclerosis (ALS) poses a significant restraint on the growth of this market. ALS is a progressive and debilitating disease that requires extensive medical care, including specialized therapies, assistive devices, and supportive care services. The high costs of these treatments and supportive care can place a substantial financial burden on patients and their families, particularly in regions with limited healthcare coverage or access to affordable treatment options.
Furthermore, the development of novel therapies for ALS often involves lengthy and resource-intensive research and clinical trials, leading to high costs for pharmaceutical companies and ultimately contributing to the overall high treatment costs for patients. This financial barrier can limit access to potentially life-changing treatments, hindering the market's growth and the ability to provide comprehensive care to all ALS patients.
Limited Approved Therapeutic Options
The limited number of approved therapeutic options for Amyotrophic Lateral Sclerosis (ALS) is a significant restraint on the market's growth. Currently, only two drugs, riluzole and edaravone (Radicava), are approved for the treatment of ALS, and both have modest effects in slowing disease progression. Despite extensive research efforts, there is still a lack of disease-modifying therapies that can effectively target the underlying causes of ALS and halt or reverse its progression.
The scarcity of approved treatments not only limits the available options for patients but also poses challenges for pharmaceutical companies and researchers in the development and commercialization of new therapies. The stringent regulatory requirements and the need for extensive clinical trials further contribute to the difficulties in bringing novel ALS treatments to market, hampering the overall growth potential of the market.
Stringent Regulatory Approval Processes
The stringent regulatory approval processes for new therapies and treatments in the Amyotrophic Lateral Sclerosis (ALS) market pose a significant restraint on its growth. Given the complex nature of ALS and the limited understanding of its underlying mechanisms, regulatory agencies have rigorous standards for evaluating the safety and efficacy of potential treatments.
Clinical trials for ALS therapies often face challenges, such as enrolling sufficient numbers of patients due to the relatively low prevalence of the disease, and demonstrating statistically significant improvements in clinical outcomes. These challenges can lead to lengthy and costly development processes, deterring pharmaceutical companies from investing in ALS research and development.
Additionally, the regulatory requirements for approval may vary across different regions, further complicating the process of bringing new treatments to market on a global scale. This stringent regulatory environment can slow down the introduction of innovative therapies and hinder the overall growth and progress of the ALS market.
Recent Developments:
|
Development |
Involved Company |
|
In September 2022, Biogen and Ionis announced positive results from a Phase 3 study of tofersen for SOD1 ALS. |
Biogen and Ionis Pharmaceuticals |
|
In August 2021, Amylyx Pharmaceuticals announced positive results from a Phase 2 trial of AMX0035 for ALS. |
Amylyx Pharmaceuticals |
|
In June 2020, the FDA approved Relyvrio (previously AMX0035) for the treatment of ALS. |
Amylyx Pharmaceuticals |
|
Product Launch |
Company Name |
|
In May 2022, Mitsubishi Tanabe Pharma launched Radicut (edaravone) for the treatment of ALS in Japan. |
Mitsubishi Tanabe Pharma |
|
In August 2021, Cytokinetics announced the FDA acceptance of its NDA for reldesemtiv for the treatment of ALS. |
Cytokinetics |
|
In May 2020, Orion Pharma launched Navitor (levosimendan) for the treatment of respiratory dysfunction in ALS. |
Orion Pharma |
|
Merger/Acquisition |
Involved Companies |
|
In April 2022, Biogen acquired Amnion Biosciences, a company developing gene therapies for ALS and other diseases. |
Biogen and Amnion Biosciences |
|
In September 2020, Ionis Pharmaceuticals acquired Akcea Therapeutics, a subsidiary focused on rare diseases. |
Ionis Pharmaceuticals and Akcea |
|
In January 2020, Biogen acquired Nightstar Therapeutics, a gene therapy company with a focus on neurodegenerative diseases. |
Biogen and Nightstar Therapeutics |
Market Regional Insights:
The Amyotrophic Lateral Sclerosis (ALS) Market is anticipated to witness significant growth across various regions due to the increasing prevalence of the disease and the development of novel treatment options.
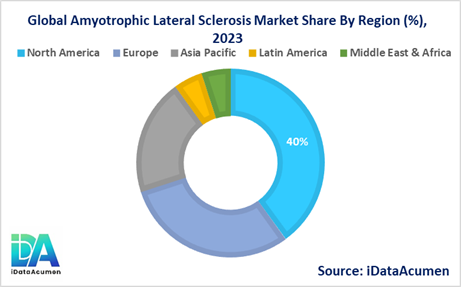
- North America is expected to be the largest market for the Amyotrophic Lateral Sclerosis (ALS) Market during the forecast period, accounting for over 40% of the market share in 2024. The growth of the market in North America is attributed to the presence of well-established healthcare infrastructure, high healthcare expenditure, and a large patient population.
- Europe is expected to be the second-largest market for the Amyotrophic Lateral Sclerosis (ALS) Market, accounting for over 30% of the market share in 2024. The growth of the market is attributed to the increasing awareness about the disease, government initiatives, and the presence of leading pharmaceutical companies engaged in research and development efforts.
- The Asia-Pacific region is expected to be the fastest-growing market for the Amyotrophic Lateral Sclerosis (ALS) Market, with a CAGR of over 10% during the forecast period by 2024. The growth of the market in the Asia-Pacific region is attributed to the improving healthcare infrastructure, rising disposable incomes, and increasing investments in healthcare research and development.
Market Segmentation:
- By Drug Class
- Riluzole
- Edaravone
- Neuroprotective agents
- Anti-inflammatory drugs
- Others
- By Route of Administration
- Oral
- Intravenous
- Intrathecal
- Subcutaneous
- Others
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Pharmacies
- Mail-order Pharmacies
- Others
- By End-User
- Hospitals
- Clinics
- Home Care Settings
- Rehabilitation Centers
- Nursing Homes
- Others
- By Disease Type
- Sporadic ALS
- Familial ALS
- Juvenile ALS
- Bulbar ALS
- Others
- By Mechanism of Action
- Glutamate Release Inhibitors
- Antioxidants
- Anti-inflammatory Agents
- Others
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East, and Africa
Segment Analysis:
- By Drug Class
- By Disease Type
- By Region
The "By Drug Class" segment is expected to witness significant growth across various regions, with the riluzole segment projected to maintain a dominant market share due to its widespread use as the only FDA-approved drug for ALS. The edaravone segment is also anticipated to experience substantial growth, driven by its recent approval and adoption in several countries.
In terms of the "By Disease Type" segment, the sporadic ALS segment is likely to remain the largest contributor to the market, as it accounts for the majority of ALS cases worldwide. However, the familial ALS segment is expected to grow at a higher CAGR due to ongoing research efforts and the potential development of targeted therapies for genetic forms of the disease.
Regionally, North America and Europe are projected to dominate the Amyotrophic Lateral Sclerosis (ALS) market, owing to factors such as well-established healthcare infrastructure, high healthcare expenditure, and a large patient population. However, the Asia-Pacific region is anticipated to witness the fastest growth, driven by improving healthcare facilities, rising disposable incomes, and increasing investments in healthcare research and development.
In 2024, the riluzole segment within the "By Drug Class" segment is expected to be the largest, followed by the edaravone segment. Additionally, the sporadic ALS segment within the "By Disease Type" segment is likely to maintain its position as the largest contributor to the market.
Top companies in the Amyotrophic Lateral Sclerosis (ALS) Market:
- Mitsubishi Tanabe Pharma Corporation
- Biogen Inc.
- Ionis Pharmaceuticals, Inc.
- Cytokinetics, Inc.
- Orion Pharma
- Apotex Inc.
- Sun Pharmaceutical Industries Ltd.
- Bausch Health Companies Inc.