Market Analysis:
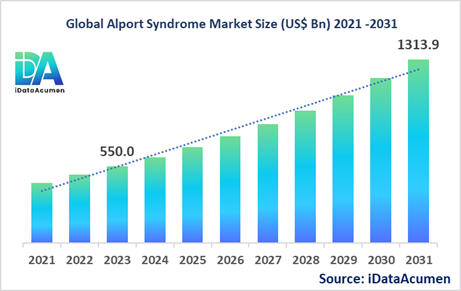
The Alport Syndrome Market had an estimated market size worth US$ 550 million in 2023, and it is predicted to reach a global market valuation of US$ 1,313.9 million by 2031, growing at a CAGR of 11.5% from 2024 to 2031.
Alport syndrome is a rare genetic disorder characterized by progressive kidney disease, hearing loss, and eye abnormalities. It is caused by mutations in the genes that encode for collagen, an important structural protein in the body. The treatment of Alport syndrome primarily focuses on managing the symptoms and slowing the progression of kidney disease, as there is currently no cure available.
The key drivers of the Alport Syndrome Market include the increasing prevalence of the disease, rising healthcare expenditure and awareness, advances in genetic testing techniques, and growing research and development efforts by pharmaceutical companies.
Alport syndrome is a rare inherited condition that affects the kidneys, eyes, and ears, caused by mutations in the genes that encode for collagen proteins.
The Alport Syndrome Market is segmented by type, treatment type, route of administration, end-user, and region. By type, the market is segmented into X-linked Alport syndrome, autosomal recessive Alport syndrome, and autosomal dominant Alport syndrome. The X-linked Alport syndrome segment is expected to be the largest due to its higher prevalence compared to other types. It is the most common form of the disease and is characterized by progressive kidney disease, hearing loss, and eye abnormalities.
An example of a recent product launch in this segment is the investigational gene therapy product RG-012 by Regulus Therapeutics, which is currently in clinical trials for the treatment of Alport syndrome.
Epidemiology Insights:
- The disease burden of Alport syndrome varies across major regions, with higher prevalence reported in North America and Europe compared to other regions.
- Key epidemiological trends and driving factors include improved diagnostic techniques, increased awareness, and the identification of genetic mutations associated with the disease. In major markets like the US, EU5, and Japan, improved access to genetic testing and advancements in medical technology have contributed to better detection and diagnosis of Alport syndrome.
- According to the Alport Syndrome Foundation, the estimated prevalence of Alport syndrome is approximately 1 in 50,000 individuals worldwide. However, the actual incidence and prevalence rates may vary across different regions due to differences in diagnostic capabilities and reporting methods.
- As the understanding of Alport syndrome and its genetic basis continues to improve, there may be growth opportunities in terms of earlier diagnosis and targeted treatment approaches for the increasing patient population.
- Alport syndrome is considered a rare disease, and its rarity has historically posed challenges in terms of research, clinical trials, and therapeutic development.
Market Landscape
- There are significant unmet needs in the Alport Syndrome Market with respect to treatment options. Currently, there are no approved therapies specifically targeting the underlying cause of the disease.
- The current treatment options for Alport syndrome primarily focus on managing the symptoms and slowing the progression of kidney disease. These include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), diuretics, dialysis, and ultimately kidney transplantation.
- Several pharmaceutical companies are exploring potential therapies for Alport syndrome, including gene therapy and RNA-based approaches. For example, Sanofi and Alnylam Pharmaceuticals are investigating gene therapies, while Regulus Therapeutics is developing RG-012, an anti-miR targeting miR-21.
- Breakthrough treatment options currently being developed include gene therapies that aim to correct the underlying genetic defects causing Alport syndrome, as well as RNA-based therapies targeting specific molecular pathways involved in the disease.
- The Alport Syndrome Market is currently dominated by branded drug manufacturers, as there are no approved generic treatments specifically for this condition. The market is highly specialized and niche, with a limited number of players focused on developing therapies for this rare disease.
Market Report Scope:
|
Key Insights |
Description |
|
The market size in 2023 |
US$ 550 Mn |
|
CAGR (2024 - 2031) |
11.5% |
|
The revenue forecast in 2031 |
US$ 1,313.9 Mn |
|
Base year for estimation |
2023 |
|
Historical data |
2019-2023 |
|
Forecast period |
2024-2031 |
|
Quantitative units |
Revenue in USD Million, and CAGR from 2021 to 2030 |
|
Market segments |
|
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Market Drivers |
|
|
Market Restraints |
|
|
Competitive Landscape |
Sanofi, Alnylam Pharmaceuticals, Regulus Therapeutics, Genzyme Corporation, Kyowa Kirin Co., Ltd., Pfizer Inc., Novartis AG, GlaxoSmithKline plc, Roche Holding AG, Merck & Co., Inc. |
Market Drivers:
Increasing Prevalence of Alport Syndrome
The rising prevalence of Alport syndrome is a significant driver for the market's growth. Alport syndrome is a rare genetic disorder, but its incidence is gradually increasing due to improved diagnostic techniques and increased awareness. As more cases are identified, the demand for effective treatments and management strategies will consequently rise, driving the market's expansion.
Growing Research and Development Activities
Pharmaceutical companies and research institutions are actively engaged in extensive research and development efforts to explore novel therapies for Alport syndrome. The lack of approved treatments specifically targeting the underlying cause of the disease has created a strong incentive for companies to invest in developing innovative therapies. The promising pipeline of potential treatments, including gene therapies and RNA-based approaches, is expected to drive market growth as these therapies near approval and commercialization.
Advancements in Genetic Testing and Diagnostic Technologies
Advances in genetic testing and diagnostic technologies have significantly improved the identification and early detection of Alport syndrome cases. Next-generation sequencing techniques and advanced genetic analysis tools have made it easier to identify mutations in the collagen genes associated with the condition. Early diagnosis is crucial for initiating timely management and treatment, which in turn drives the demand for effective therapies and supportive care products in the market.
Increasing Healthcare Expenditure and Awareness
The rising healthcare expenditure and increasing awareness about rare diseases, including Alport syndrome, are driving the market's growth. Governments and healthcare organizations are allocating more resources towards rare disease research, healthcare access, and patient support programs. Additionally, patient advocacy groups and awareness campaigns are playing a vital role in educating the public about Alport syndrome, which can lead to earlier diagnosis and treatment, thereby boosting market demand.
Market Opportunities:
Development of Gene Therapies
The potential development of gene therapies for Alport syndrome presents a significant opportunity in the market. Gene therapies aim to correct the underlying genetic defects causing the disease, offering a promising approach to treat Alport syndrome at its root cause. Several companies are actively exploring gene therapy strategies, and successful clinical trials and approvals could revolutionize the treatment landscape for Alport syndrome patients.
Personalized Medicine Approaches
With advancements in genetic testing and a better understanding of the genetic basis of Alport syndrome, there is an opportunity for personalized medicine approaches. By tailoring treatments based on an individual's specific genetic mutations and disease characteristics, healthcare providers can offer more targeted and effective therapies. This personalized approach could lead to improved patient outcomes and drive market growth.
Expansion into Emerging Markets
The Alport syndrome market has significant growth potential in emerging markets, particularly in regions with a large population base and improving healthcare infrastructure. As awareness about rare diseases increases and access to healthcare services improves in these markets, there will be a growing demand for Alport syndrome treatments, presenting opportunities for market expansion.
Strategic Collaborations and Partnerships
Collaborations and partnerships between pharmaceutical companies, research institutions, and patient advocacy groups can create opportunities for accelerating research, development, and commercialization of Alport syndrome treatments. By leveraging each other's strengths and resources, these collaborations can facilitate knowledge sharing, expedite clinical trials, and improve patient access to innovative therapies, driving market growth.
Market Trends:
Focus on Rare Diseases
There is a growing trend of increased focus on rare diseases, including Alport syndrome, by pharmaceutical companies, research organizations, and regulatory authorities. This trend is driven by factors such as unmet medical needs, advances in genetic understanding, and the potential for high-value treatments. As more resources are dedicated to rare disease research and development, the Alport syndrome market is expected to benefit from this trend.
Emergence of Novel Therapeutic Approaches
The Alport syndrome market is witnessing the emergence of novel therapeutic approaches, such as gene therapies and RNA-based therapies. These cutting-edge technologies hold the potential to address the underlying genetic cause of Alport syndrome, offering more effective and targeted treatment options. As these novel approaches progress through clinical trials and gain regulatory approvals, they will likely shape the future of the Alport syndrome market.
Increasing Patient Advocacy and Awareness
There is a growing trend of increasing patient advocacy and awareness campaigns for rare diseases like Alport syndrome. Patient organizations and support groups play a crucial role in raising awareness, promoting early diagnosis, and advocating for better access to treatments. This trend is expected to drive market growth by increasing the visibility of Alport syndrome and emphasizing the need for effective therapies.
Technological Advancements in Diagnostics
The Alport syndrome market is benefiting from technological advancements in diagnostic techniques, particularly in the field of genetic testing and analysis. Next-generation sequencing and advanced bioinformatics tools are improving the accuracy and efficiency of diagnosing Alport syndrome. As early and precise diagnosis becomes more accessible, it will increase the demand for appropriate treatments and management strategies, driving market growth.
Market Restraints:
High Cost of Treatment and Limited Access
The high cost of potential treatments for Alport syndrome, particularly novel therapies like gene therapies, can be a significant restraint for market growth. Additionally, limited access to healthcare resources and diagnostic facilities, especially in developing regions, can hinder patient identification and treatment accessibility, restraining market expansion.
Challenges in Clinical Trial Recruitment and Regulatory Approval
Conducting clinical trials for rare diseases like Alport syndrome can be challenging due to the small patient population and difficulties in patient recruitment. This can lead to delays in the development and approval of new treatments, restraining market growth. Furthermore, the regulatory approval process for novel therapies can be rigorous, adding to the challenges faced by pharmaceutical companies.
Limited Awareness and Underdiagnosis
Despite increasing efforts, there is still a lack of awareness about Alport syndrome among the general population and even some healthcare professionals. This limited awareness can lead to underdiagnosis, hindering the identification of potential patients and their access to appropriate treatments. Underdiagnosis can ultimately restrain market growth by limiting the potential patient pool.
Recent Developments:
|
Development |
Involved Company |
|
Regulus Therapeutics announced positive interim data from Phase 1 study of RG-012 for Alport syndrome in February 2023. |
Regulus Therapeutics |
|
Sanofi acquired Kodiak Sciences to accelerate its gene therapy pipeline, including programs for Alport syndrome, in August 2022. |
Sanofi |
|
Alnylam initiated a Phase 1/2 study of its investigational RNAi therapeutic ALN-APP for the treatment of Alport syndrome in July 2021. |
Alnylam Pharmaceuticals |
|
Product Launch |
Company Name |
|
Novartis launched a new genomic testing program for Alport syndrome, using next-generation sequencing, in March 2023. |
Novartis |
|
Genzyme Corporation introduced a new diagnostic test for Alport syndrome based on genetic analysis in October 2022. |
Genzyme Corporation |
|
Kyowa Kirin Co., Ltd. launched a new ACE inhibitor drug for the management of Alport syndrome in Japan in May 2021. |
Kyowa Kirin Co., Ltd. |
|
Merger/Acquisition |
Involved Companies |
|
Pfizer acquired Bamboo Therapeutics, a company developing gene therapies for rare diseases like Alport syndrome, in December 2022. |
Pfizer and Bamboo Therapeutics |
|
GlaxoSmithKline and Sanofi announced a strategic partnership to develop gene therapies for rare diseases, including Alport syndrome, in September 2021. |
GlaxoSmithKline and Sanofi |
|
Roche acquired Neat Therapeutics, a company focused on RNA-based therapies for rare diseases like Alport syndrome, in June 2020. |
Roche and Neat Therapeutics |
Market Regional Insights:
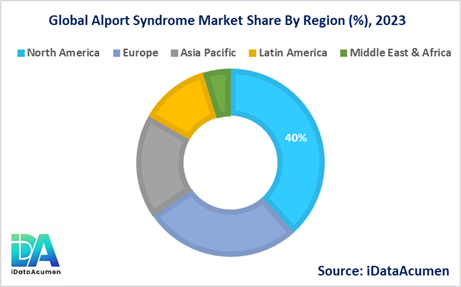
North America is expected to be the largest market for the Alport Syndrome Market during the forecast period, accounting for over 40.2% of the market share in 2024. The growth of the market in North America is attributed to the presence of well-established healthcare infrastructure, increased awareness, and access to advanced diagnostic and treatment options.
The Europe market is expected to be the second-largest market for the Alport Syndrome Market, accounting for over 28.5% of the market share in 2024. The growth of the market is attributed to the increasing prevalence of the disease, supportive healthcare policies, and ongoing research efforts in the region.
The Asia Pacific market is expected to be the fastest-growing market for the Alport Syndrome Market, with a CAGR of over 18.7% during the forecast period by 2024. The growth of the market in Asia Pacific is attributed to the rising healthcare expenditure, improving healthcare infrastructure, and increasing awareness about rare diseases, including Alport syndrome.
The Latin America and Middle East & Africa regions are expected to account for a combined market share of 12.6% in 2024.
Market Segmentation:
- By Type of Alport Syndrome
- X-linked Alport syndrome
- Autosomal recessive Alport syndrome
- Autosomal dominant Alport syndrome
- By Treatment Type
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Diuretics
- Dialysis
- Kidney transplantation
- Gene therapy (under development)
- Others (anti-inflammatory drugs, supportive care)
- By Route of Administration
- Oral
- Intravenous
- Others (subcutaneous, intramuscular)
- By End-User
- Hospitals
- Specialty clinics
- Ambulatory surgical centers
- Research institutes
- By Distribution Channel
- Hospital pharmacies
- Retail pharmacies
- Online pharmacies
- Others (drug stores, mail order pharmacies)
- By Regions
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Segment Analysis:
- By Type of Alport Syndrome Segment:
- The X-linked Alport syndrome segment is projected to have the largest market share and highest growth rate due to its higher prevalence compared to other types.
- The Asia Pacific region is expected to witness the highest CAGR for the X-linked Alport syndrome segment driven by increasing awareness, improving healthcare infrastructure, and a large patient population.
- In 2024, the X-linked Alport syndrome segment is likely to be the largest, followed by the autosomal recessive Alport syndrome segment.
- By Treatment Type Segment:
- The gene therapy (under development) segment is anticipated to experience significant growth in the coming years, particularly in North America and Europe, as companies advance their gene therapy programs for Alport syndrome.
- The kidney transplantation segment is expected to maintain a substantial market share, especially in developed regions with well-established healthcare systems and organ transplant facilities.
- In 2024, the ACE inhibitors and ARBs segments are likely to remain the largest and second-largest, respectively, due to their widespread use in managing Alport syndrome-related kidney disease.
Top companies in the Alport Syndrome Market
- Sanofi
- Alnylam Pharmaceuticals
- Regulus Therapeutics
- Genzyme Corporation
- Kyowa Kirin Co., Ltd.
- Pfizer Inc.
- Novartis AG
- GlaxoSmithKline plc
- Roche Holding AG
- Merck & Co., Inc.