Adeno-Associated Virus (AAV) Vectors in Gene Therapy Market Expected to Achieve US$ 8.9 Bn by 2031 with a CAGR 28.5% from 2023 to 2031. The Adeno-Associated Virus (AAV) Vectors in Gene Therapy Market is a part of the biopharmaceutical industry, focusing on developing and manufacturing viral vectors used in gene therapy treatments. Gene therapy is an emerging and rapidly growing field, aiming to treat or prevent genetic disorders by introducing functional genes into the body to correct or replace defective ones.
The AAV vectors market is driven by the increasing prevalence of genetic disorders, advancements in gene therapy research, and the growing demand for personalized medicine. AAV vectors are particularly attractive due to their non-pathogenic nature, ability to infect both dividing and non-dividing cells, and long-term gene expression capabilities. These factors, combined with the ongoing development of new gene therapies targeting a wide range of diseases, are fueling the market's growth.
The market's expansion is further propelled by the increasing number of clinical trials and regulatory approvals for gene therapies utilizing AAV vectors. Significant investments from pharmaceutical companies and research institutions in gene therapy research and development are also contributing to the market's growth.
Market Growth, Market Dynamics, Market Opportunity Assessment
The Adeno-Associated Virus (AAV) Vectors in Gene Therapy Market is witnessing remarkable growth due to several factors. Firstly, the increasing prevalence of genetic disorders, such as Duchenne muscular dystrophy, hemophilia, and spinal muscular atrophy, is driving the demand for effective gene therapies. As the understanding of the genetic basis of these diseases advances, the potential for targeted gene therapy treatments using AAV vectors increases.
Secondly, the market is benefiting from the rapid advancements in gene therapy research and the development of new AAV vector technologies. Improvements in vector engineering, such as the development of novel serotypes and capsid modifications, have enhanced the efficiency and specificity of gene delivery, further fueling market growth.
Additionally, the growing adoption of personalized medicine and the shift towards targeted therapies have created a favorable environment for the AAV vectors market. Gene therapies offer the potential for tailored treatments based on an individual's genetic profile, aligning with the goals of personalized medicine.
The market dynamics are also influenced by regulatory initiatives and supportive policies. Regulatory authorities, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines and streamlined pathways for the approval of gene therapies, providing a clear framework for manufacturers and facilitating market growth.
Furthermore, the market presents significant opportunities for innovation and collaboration. Strategic partnerships between biotechnology companies, research institutions, and academic centers are fostering the development of novel AAV vector technologies and gene therapy approaches. These collaborations are expected to drive further advancements in the field, leading to the development of more effective and targeted gene therapies for a wider range of diseases.
Drivers:
- Increasing Prevalence of Genetic Disorders: The rising incidence of genetic disorders, such as hemophilia, cystic fibrosis, and muscular dystrophies, is driving the demand for effective gene therapies, consequently boosting the AAV vectors market.
- Advancements in Gene Therapy Research: Continuous research and development in gene therapy have led to improved understanding of disease mechanisms, vector engineering, and gene delivery methods, enabling more efficient and targeted gene therapies using AAV vectors.
Trends:
- Development of Novel AAV Serotypes and Capsid Engineering: Researchers are focusing on developing new AAV serotypes and engineering capsids to improve tissue specificity, enhance transduction efficiency, and reduce immune responses.
- Increasing Focus on Rare Genetic Diseases: There is a growing emphasis on developing gene therapies targeting rare genetic disorders, as these conditions often have limited treatment options and high unmet medical needs.
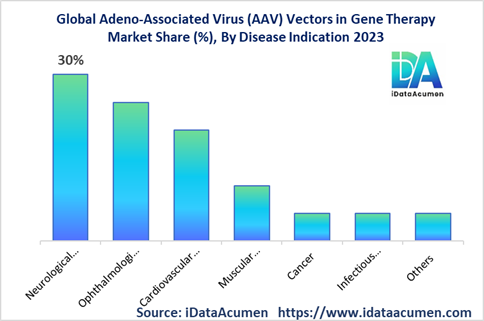
Market Opportunity: Expanding the application of AAV vectors in various therapeutic areas, such as neurological disorders, cardiovascular diseases, and cancer, presents significant market opportunities.
Key Report Insights:
- Largest Region: North America, with a market share of 42%. Prominent companies in the region include Spark Therapeutics, Biomarin Pharmaceutical, and Askbio. The region's market growth is driven by factors such as a well-established healthcare infrastructure, supportive regulatory environment, and substantial investments in gene therapy research.
- Second Largest Region: Europe, with a market share of 30%. Companies like UniQure, Oxford Biomedica, and Orchard Therapeutics have a strong presence in the region. Europe's market growth is fueled by advancements in gene therapy research, increasing awareness, and favorable reimbursement policies for innovative therapies.
- Prominent Companies in the Market: Novartis, Sanofi, Audentes Therapeutics, Pfizer, Regenxbio, and Astellas Gene Therapies. These companies are actively involved in developing gene therapies using AAV vectors and have a strong product pipeline and collaborations with research institutions.
Adeno-Associated Virus (AAV) Vectors in Gene Therapy Market Segmentation:
- By Vector Type
- AAV1
- AAV2
- AAV5
- AAV6
- AAV8
- AAV9
- Others (AAV3, AAV4, AAV7, AAV10, etc.)
- By Disease Indication
- Neurological Disorders
- Ophthalmological Disorders
- Cardiovascular Disorders
- Muscular Disorders
- Cancer
- Infectious Diseases
- Others (Metabolic Disorders, Hematological Disorders, etc.)
- By Gene Type
- Antigen
- Cytokine
- Tumor Suppressor
- Suicide Gene
- Deficiency Gene
- Others (Growth Factors, Receptors, etc.)
- By Route of Administration
- Intravenous
- Intramuscular
- Subretinal
- Intrathecal
- Others (Intraperitoneal, Intranasal, etc.)
- By End-User
- Hospitals
- Clinics
- Research Institutes
- Pharmaceutical and Biotechnology Companies
- Others (Academic Institutions, Contract Research Organizations, etc.)
- By Manufacturing Process
- Adherent Cell Culture
- Suspension Cell Culture
- Others (Transgenic Production, Insect Cell Culture, etc.)
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Definition:
The Adeno-Associated Virus (AAV) Vectors in Gene Therapy Market refers to the industry involved in the development, manufacturing, and commercialization of gene therapies that utilize adeno-associated viruses (AAVs) as vectors for delivering therapeutic genes into target cells. AAVs are small, non-pathogenic viruses that can efficiently infect both dividing and non-dividing cells, making them suitable vehicles for gene delivery. These gene therapies aim to treat various genetic disorders and acquired diseases by introducing functional genes into cells to correct or replace faulty or missing genes responsible for the disease.
The AAV vectors in gene therapy market encompasses companies engaged in research and development, clinical trials, manufacturing, and distribution of AAV-based gene therapies. These therapies hold the potential to address a wide range of diseases, including neurological disorders, muscular dystrophies, hemophilia, retinal diseases, and certain cancers, among others. The market involves the development of novel AAV serotypes, gene editing technologies, and advancements in manufacturing processes to enhance the efficacy, safety, and accessibility of these cutting-edge treatments.